Learning Objectives: What are Proteins? How Proteins are Formed? What is Peptide Bond? Peptide vs Protein, C-Terminal and N-Terminal of Proteins, pI of Proteins, Sub-units of Proteins and Characteristics of Peptide Bond
What are Proteins?
Ø Proteins are the polymers of amino acids (specifically the polymers of L-α- amino acids).
Ø During protein synthesis (translation), the amino acids are joined one by one covalently through the peptide bond.
Ø The sequence of amino acids in a protein is determined by the genetic sequence which is encoded in the genetic code.
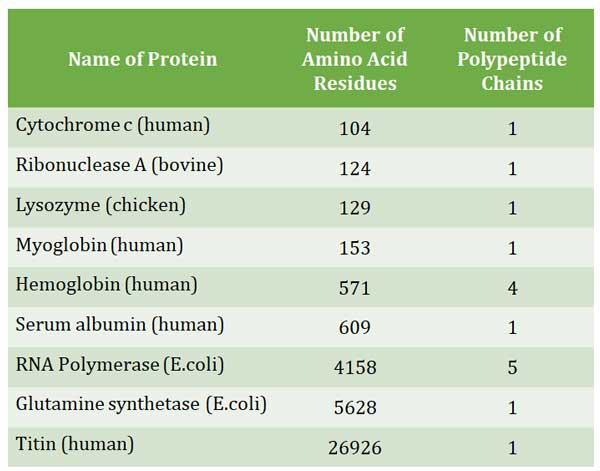
Ø The size of the protein ranges from three to thousands of amino acids (see the table below)
Ø Proteins are the most abundant organic molecules in the living system.
Ø Proteins occur in every part of the cell and they constitute about 50% of the cellular dry weight.
Ø Protein is one among the four macro-molecules (Carbohydrates, Lipids, Proteins and Nucleic Acids) of the cells and they form the fundamental basis of the structure and function of life.
Ø Protein was first described by Gerardus Johannes Mulder and the term was coined by Jöns Jacob Berzelius in 1838.
Ø The term PROTEIN was derived from the Greek word ‘proteios‘ meaning ‘holding the first place’ highlighting the prime importance of proteins in the living system.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Ø Mulder used the term proteins for the high molecular weight nitrogen-rich and most abundant substances present in animals and plants.
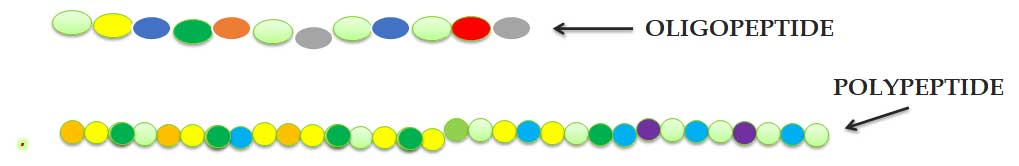
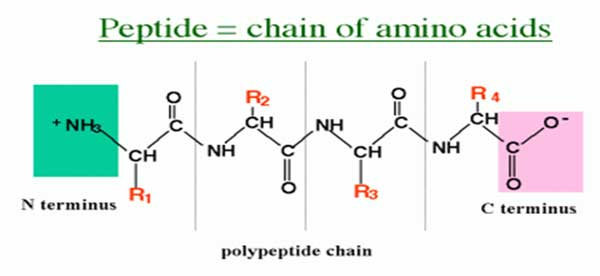
Dipeptide, Tripeptide, Oligopeptide, Polypeptide
Ø TWO amino acids joined through ONE peptide bond to form a DIPEPTIDE.
Ø THREE amino acids joined through TWO peptide bonds to form a TRIPEPTIDE.
Ø A few amino acids are joined to form an OLIGOPEPTIDE.
Ø Many amino acids are joined to form a POLYPEPTIDE.
Ø The amino acid molecules in a peptide are called residues.
Peptide vs Protein
Ø Word polypeptide and peptide are a little ambiguous and can overlap in meaning.
Ø Protein is generally used to refer to the complete biological molecule in a stable conformation.
Ø Whereas peptide is generally reserved for a short amino acid oligomer often lacking a stable 3D structure.
Ø The boundary between the two is not well defined and usually lies near 20–30 residues
Ø A polypeptide can refer to any single linear chain of amino acids, usually regardless of length, but often implies an absence of a defined conformation and function.
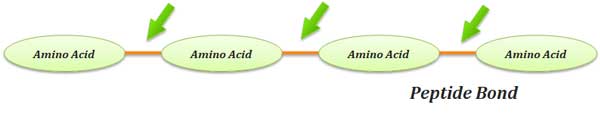
What is Peptide Bond?
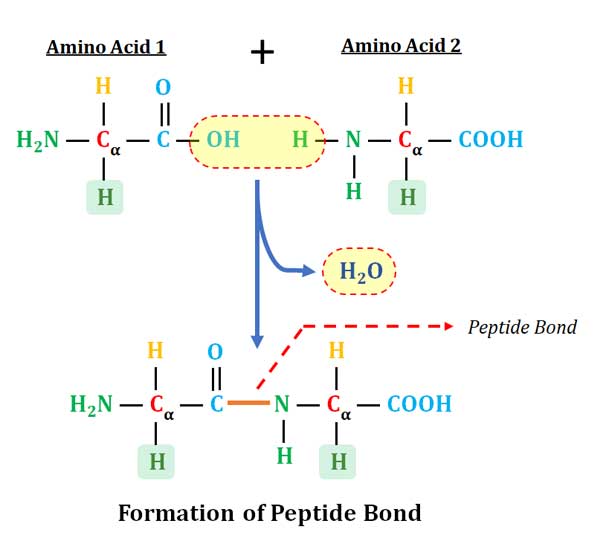
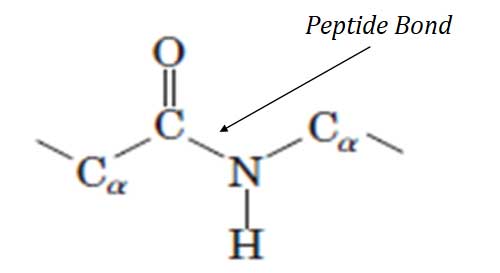
Ø Definition: A peptide bond is a covalent bond joining the α-amino group of one amino acid to the carboxyl group of another with the loss of a water molecule.
Ø In proteins, the amino acids are covalently joined through a substituted amide linkage called PEPTIDE BOND.
Ø The peptide bond is formed by the removal of ONE molecule of water from the α –COOH of one amino acid and α –NH2 of another amino acid.
Ø Peptide bond formation is an example of a condensation reaction (a very common reaction in the living system).
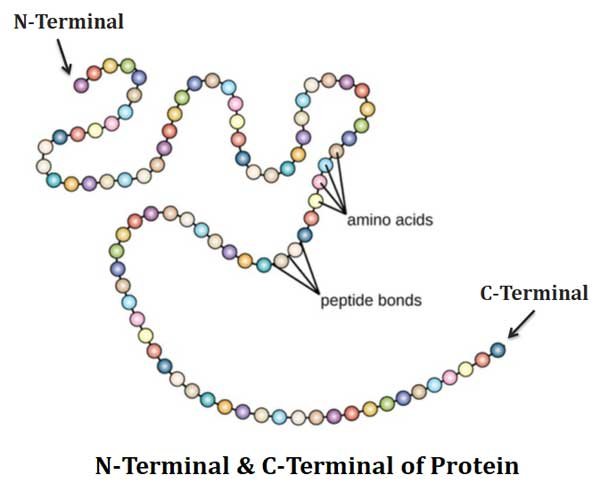
N – Terminal and C – Terminal of Protein
Ø Proteins are usually open chain forms and possess two ends called terminals on either side.
Ø In a peptide, the amino acid residue at the end with a free α amino group is called the amino terminal or N – TERMINAL.
Ø The residue at the other end which has a free α- carboxyl group is called carboxyl terminal or C – TERMINAL.
The pI of Proteins
Ø Amino groups and carboxyl groups of all non-terminal amino acids in a protein are involved in peptide bonds.
Ø These amino acids which are involved in peptide bonds do NOT ionize. Thus, they do NOT contribute to the total acid or base properties of a polypeptide.
Ø However, each polypeptide has one free carboxyl group and one amino group at their C-terminal and N-terminal respectively.
Ø These ends can ionize as free amino acids.
Ø Some amino acids in proteins have ionizable side chains (R groups) such as basic amino acids (Lysine, arginine and histidine) and acidic amino acids (glutamic acid and aspartic acid).
Ø The ionizable groups in the side chain (R groups) contribute to the overall acid or base properties of a protein.
Ø Thus, like amino acids, a polypeptide also has an isoelectric pH (pI).
Ø At pI the proteins do NOT move in the electric field.
Ø This principle is used in the protein purification technique called isoelectric focusing.
Sub-units of Protein
Ø Some protein consists of a single polypeptide chain.
Ø Some others contain multi-subunits having many peptides.
Ø The individual peptides in a functional protein may be identical or different.
Ø Example: Hemoglobin has FOUR polypeptide chains, TWO identical α chains and TWO identical β chains.
Characteristics of Peptide Bond
Ø The peptide bond is an amide linkage and it is rigid and planar.
Ø The peptide bond is a SINGLE bond but it has a partial double bond character.
Ø Due to the partial double bond character, free rotation is NOT permitted around the peptide bond.
Ø The α carbon atom in adjacent amino acid residues is separated by three covalent bonds, arranged as Cα – C – N – Cα.
Ø The peptide C – N bond is shorter than the C – N bonds in simple amines.
Ø Also, atoms associated with the peptide bond are co-planar.
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
Ø There is a resonance or partial sharing of two pairs of electrons between the carbonyl oxygen and the amide nitrogen in the peptide bond.
Ø The oxygen has a partial negative charge and the nitrogen has a partial positive charge resulting in a small eclectic dipole.
Ø Thus, the C – N bond is unable to rotate freely because of its partial double bond character.
Ø Rotation is permitted only at N – Cα and Cα – C bonds.
Ø The SIX atoms associated with the peptide bond are in a single plane with the oxygen atom of the carbonyl group and the hydrogen atom of the amide nitrogen trans to each other.
Summary: Proteins are the polymers of amino acids. Amino acids are joined through a special type of covalent bond called the peptide bond. The peptide bond is a substituted amide linkage formed between the amino group of one amino acid and the carboxylic group of the second amino acid. The peptide bond has a partial double bond character hence free rotation is not permitted around the peptide bond even if it is a single bond.
Try this QUIZ on Peptide Bond
Hope this post was helpful to understand the Concept of Protein and the Characteristics of Peptide Bonds. We would like to hear your feedback and suggestions.
Please comment (below ↓)
<<< Back to BIOCHEMISTRY Home Page
You may also like.
@. Classification of Amino Acids
Reference:
David, L., Nelson, D.L., Cox, M.M., Stiedemann, L., McGlynn Jr, M.E. and Fay, M.R., 2000. Lehninger principles of biochemistry.
Voet, D. and Voet, J.G., 2010. Biochemistry. John Wiley & Sons.
Key Questions on Proteins and Peptide Bond
- What are proteins?
- Difference between Peptide and Protein
- How peptide bond is formed?
- What are the N-terminal and C-terminal of a Protein?
- Do proteins have pI (isoelectric pH)?
- What are the characteristics of peptide bonds?
- What is the reason for the partial double bond character of peptide bonds?



good