In the previous post, we discussed the Structure of Amino acids. There we have discussed the characteristics of amino acids and their ionic behavior. Here, we will discuss Classification of Amino Acids in Biochemistry. Amino acids are classified based on different criterions such as properties of R-group, nutrition, metabolic fate and charge. We will look into each system with its characteristics and examples. The best method to classify amino acids is based on the properties of the R group.
How Amino Acids are Classified?
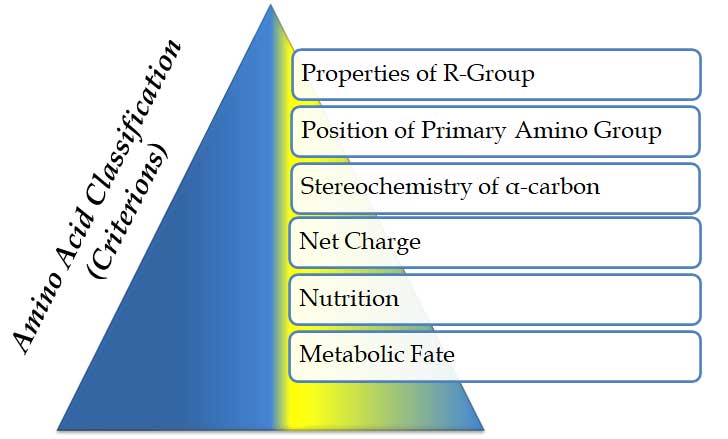
Ø Amino acids are classified based on MANY criteria. These criteria are listed below
(A). Properties of R-group
(B). Position of the primary amino group
(C). Stereochemistry of α-carbon atom
(D). Based on net-charge
(E). Based on nutrition (essentiality)
(F). Based on the metabolic fate
Classification of Amino Acids
(A). Classification based on the Properties of R-group
Ø The R-group is the side chain of an amino acid (for details see Structure of Amino Acids). In the previous post, as we explained the structure of amino acids, the R-group is the only part that varies in all 22 proteogenic amino acids.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Ø Thus, the best classification of amino acids is the one that is based on the properties of R-groups.
Ø Different criteria/properties of R-groups that we consider for the classification of amino acids are:
$. The Polarity of R groups
$. Aliphatic or Aromatic nature of R groups
$. Acidic or Basic nature of R groups
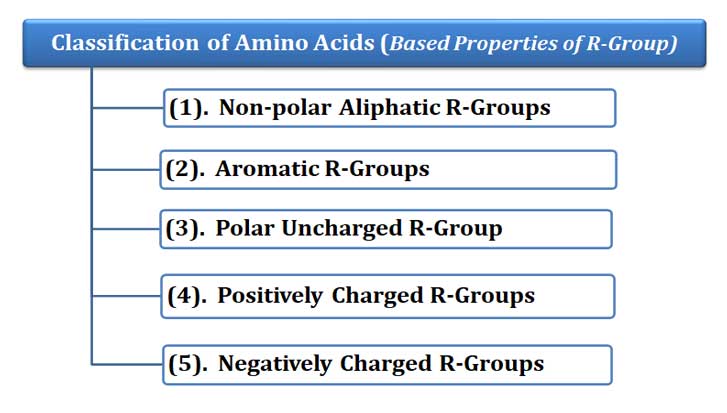
Ø Based on the above-mentioned properties of R groups, all proteogenic amino acids are grouped into FIVE classes.
Ø They are:
(1). Non-polar aliphatic R-groups
(2). Aromatic R-groups
(3). Polar uncharged R-groups
(4). Positively charged (Basic) R-groups
(5). Negatively charged (Acidic) R-groups
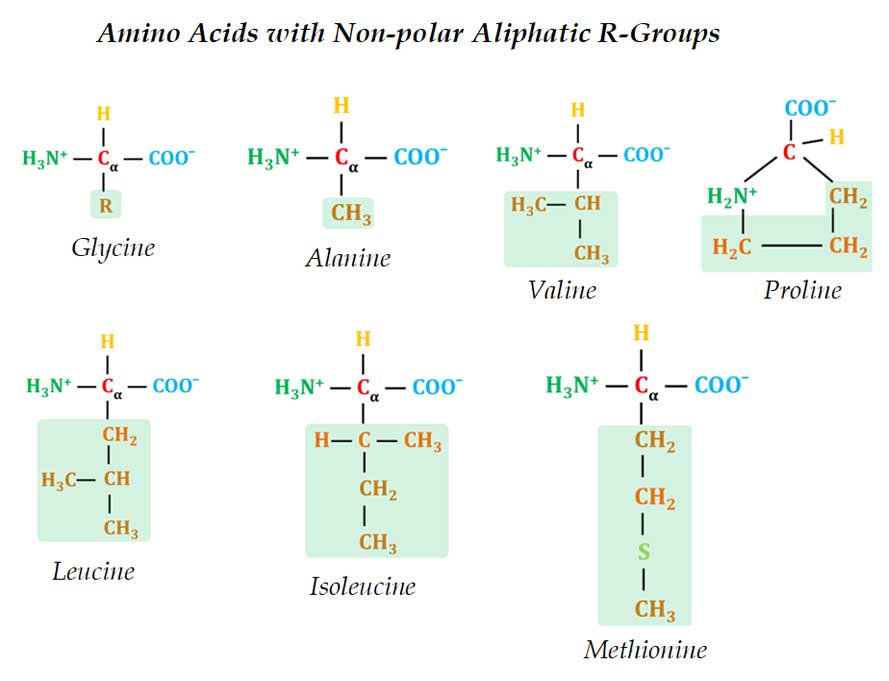
(1). Non-polar Aliphatic R-Groups
Ø Here the R-groups are non-polar and hydrophobic.
Ø Examples: Glycine, Alanine, Proline, Valine, Leucine, Isoleucine & Methionine
Ø All these amino acids are non-polar and hydrophobic.
Ø They are completely insoluble in water.
Ø Glycine is the simplest amino acid; the side chain is – H.
Ø Glycine is the only proteogenic amino acid with an achiral α-carbon (symmetric α-carbon and hence no D and L forms in Glycine).
Ø Due to the small size of glycine, it is often abundantly found in the turns and loops of polypeptides (For details, please visit Protein Structure).
Ø The side chain of alanine is a methyl group (-CH3).
Ø In valine, the side chain is isopropyl group (-CH – (CH3)2)
Ø In leucine, the side chain is the isobutyl group (CH2 – CH – (CH3)2)
Ø In isoleucine, the R-group is a branched hydrocarbon chain.
Ø Proline is an unusual amino acid with a distinctive cyclic structure.
Ø The amino group of the Proline is held in a rigid conformation with the R group.
Ø Thus, Proline is structurally an ‘Imino acid’.
Ø The cyclic structure of Proline reduces the rotational flexibility of polypeptides.
Ø This is why Proline is causing a bend in the polypeptide chain.
Ø Methionine is one of the sulfur-containing amino acids.
Ø Methionine has a nonpolar S-methyl thioether group in the side chain.
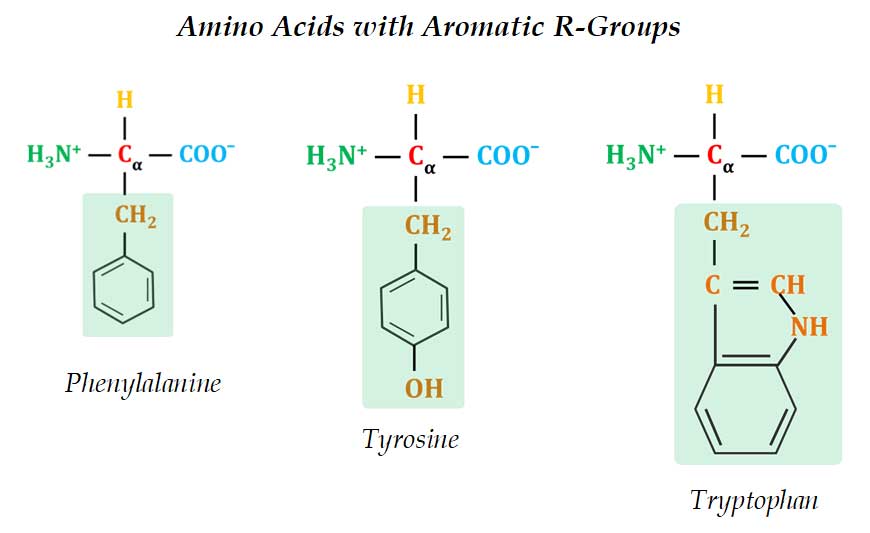
(2). Aromatic R-Groups
Ø These amino acids have aromatic side chain which is usually non-polar or slightly polar.
Ø Examples: Phenylalanine, Tyrosine and Tryptophan
Ø The aromatic side chains are relatively non-polar and hydrophobic.
Ø All can contribute to hydrophobic & hydrophilic interactions with water.
Ø Phenylalanine is completely non-polar due to the benzyl side chain.
Ø The side chain of Tyrosine is composed of a tyrosyl functional group (a benzyne ring with one -OH).
Ø The –OH group of the Tyrosine can form hydrogen bonds and thus, Tyrosine forms an important functional group in the active sites of some enzymes.
Ø The side chain of Tryptophan is an indole ring (a six-membered benzene ring fused to a five-membered pyrrole ring).
Ø Tyrosine and Tryptophan are more polar than phenylalanine due to the presence of the hydroxyl group and nitrogen group respectively.
Ø Tryptophan and Tyrosine can absorb ultraviolet light at 280 nm.
Ø This is why proteins show a characteristic absorption peak at 280 nm.
Ø Thus, for the quantification of proteins by spectrophotometry 280 nm wavelength is used. (Please note for the quantification of DNA and RNA the wavelength used is 260 nm. The 260/280 ratio is often used for testing the quality of isolated DNA or RNA. For details, please visit Biotechnology Notes Page)
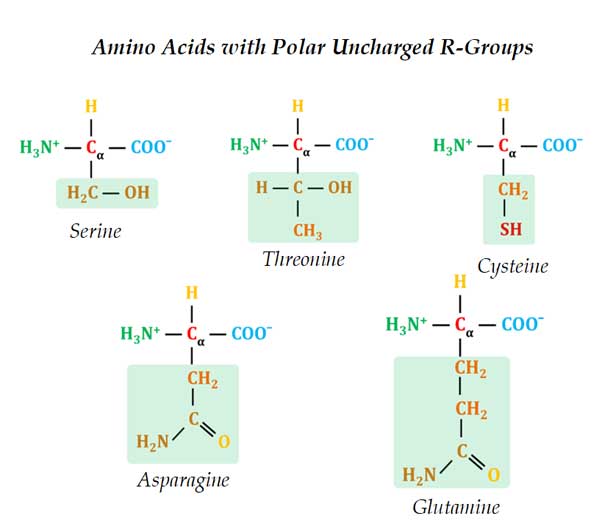
(3). Polar Uncharged R-Groups
Ø These amino acids are polar and thus they are soluble in water.
Ø However, their side chain does NOT contain any charged groups.
Ø Examples: Serine, Threonine, Cysteine, Asparagine & Glutamine
Ø All are hydrophilic since they contain functional groups that can form hydrogen bonds with water
Ø The polarity in these amino acids is provided by:
o Serine & Threonine: Hydroxyl group
o Cysteine: Sulfhydryl group (SH group)
o Asparagine & Glutamine: Amide group
Ø The -OH groups of the side chains of Serine and Threonine are the site of O-linked glycosylation (for details please see Glycoconjugates). They can also act as the site of the phosphorylation of proteins.
Ø Asparagine and Glutamines are the amides of TWO other amino acids – Aspartate and Glutamate respectively.
Ø The amide nitrogen of asparagine act as the site of N-linked glycosylation.
Ø Cysteine is a Sulfur-containing amino acid. The sulfur is present as a highly polar sulfhydryl group (SH group)
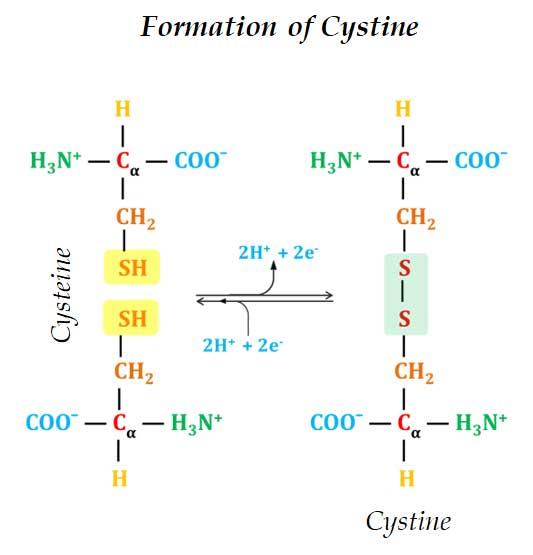
Ø Cysteine is readily oxidizable.
Ø Two Cysteine molecules oxidize to form a covalently linked dimeric amino acid called Cystine.
Ø In Cystine two Cysteine molecules are joined by a disulfide bond.
Ø The disulfide bonds are strongly hydrophobic (nonpolar).
Ø Disulfide bonds play a crucial role in the structure of proteins.
Ø Disulfide bond forms covalent bonds between amino acids in the same polypeptide chains (intra) or between different polypeptide chains (inter) (for details please see Bonds Involved in Protein Structure).
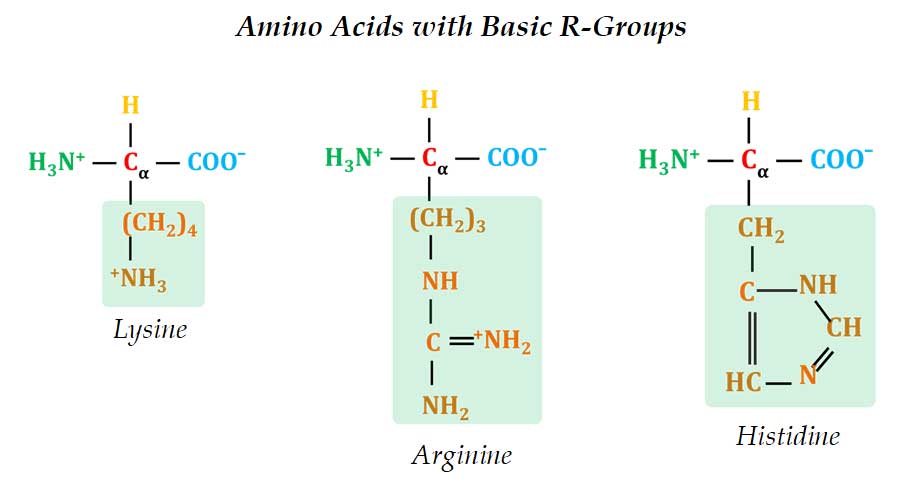
(4). Positively Charged R-Groups
Ø Most hydrophilic R-groups are those with + ive or – ive charged groups.
Ø In positively charged amino acids, the R group contain basic groups.
Ø These amino acids are collectively called Basic Amino Acids.
Ø Example: Lysine, Arginine and Histidine
Ø Lysine has a secondary amino group at the ε (epsilon) position.
Ø Arginine has a positively charged guanidino group.
Ø Histidine has an imidazole ring.
Ø Histidine is the only amino acid having an ionizable side chain with pKa near neutral pH (pH 7.0) or at biological pH (7.4).
Ø This is why most enzymes’ catalytic sites have one or many Histidine residues that serves as proton donors or acceptors in enzymatic reactions. Example RNases
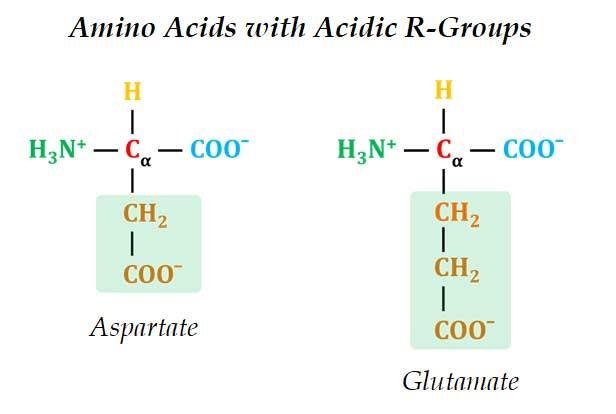
(5). Negatively Charged R-Groups
Ø In negatively charged amino acids, the side chain contains acidic R groups.
Ø Example: Aspartate (Aspartic acid) and Glutamate (Glutamic acid)
Ø Both have a second carboxyl group in the side chain.
Ø The extra carboxyl group contribute an extra negative charge.
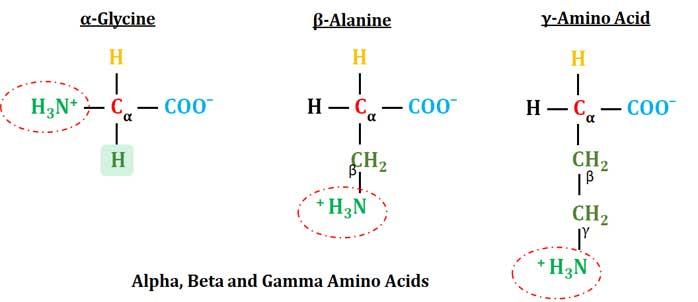
(B). Classification based on the Position of Primary Amino Group
Ø Based on the core structure of an amino acid (location of NH2 group) the amino acids can be classified as:
Ø α-Amino Acid: The primary amino group is attached to the α-carbon atom. All proteogenic amino acids are α-amino acids. For details, please visit Structure of Amino Acids.
Ø β-Amino Acid: Primary amino group is attached to the second carbon atom (β-carbon).
Ø γ-Amino Acid: Primary amino group is attached to the γ carbon.
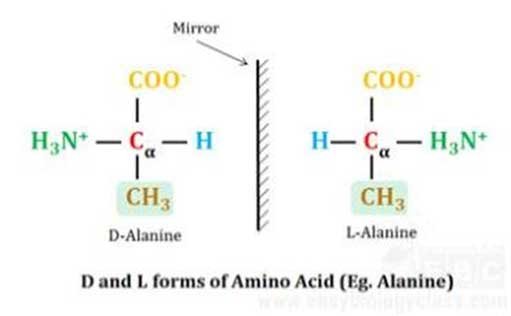
(c). Classification based on stereochemistry of α-carbon atom
Ø Based on the stereochemistry of the α-carbon atom, amino acids can exist in two different stereoisomeric forms.
Ø Except for glycine, the α-carbon atom of all proteogenic amino acids is asymmetric (the vacancies are filled by four different groups).
Ø Thus, stereoisomers are possible around these central carbon atoms and they are named D- L- isomers. Nomenclature is similar to that of monosaccharides (For details please see Monosaccharides).
Ø All protein-coding natural amino acids are L-isomers (Structure of Amino Acids).
(D). Classification based on Net Charge
Ø This classification is based on the presence or absence of extra-charged groups in the side chains of amino acids.
Ø This category can be further grouped into Positively charged amino acids (Eg. Glutamic Acid and Aspartic Acid), Negatively charged amino acids (Eg. Lysine, Arginine and Histidine) and amino acids with no charge (in the side chain) (Eg. Valine).
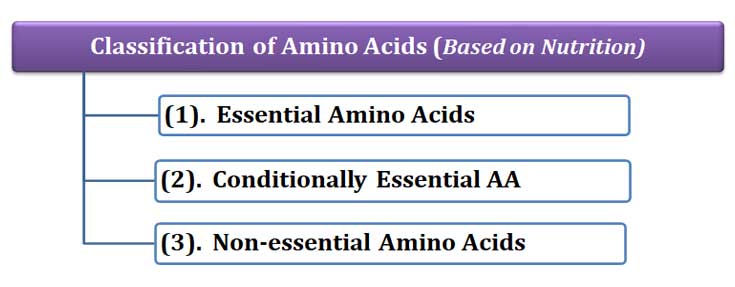
(E). Classification based on Nutrition
Ø Based on the nutritional requirements of amino acids, there can be three classes of amino acids. They are:
(1). Essential amino acids
(2). Conditionally essential amino acids
(3). Non-essential amino acids
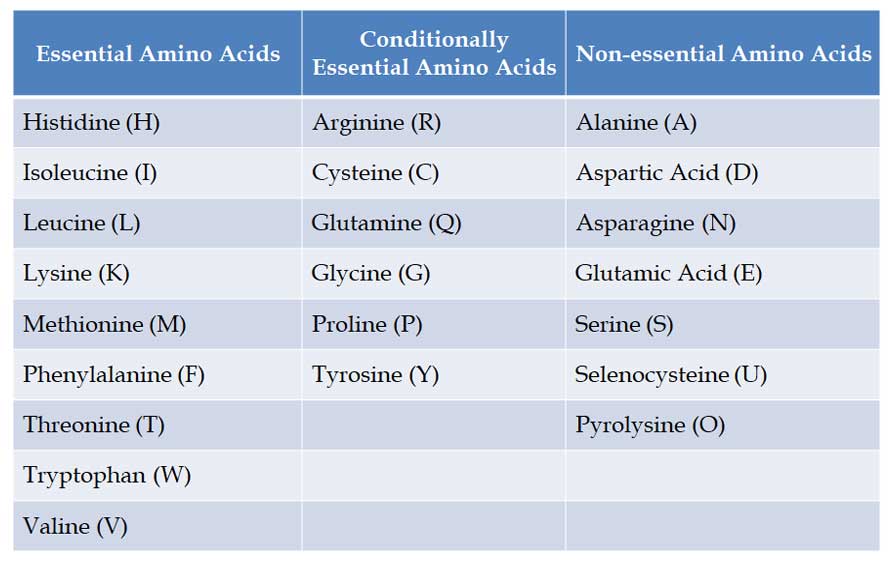
(1). Essential Amino Acids
Ø Definition: Essential amino acids cannot be made by the human body and they must come from the diet.
Ø NINE Essential Amino Acids to humans: Phenylalanine, Valine, Threonine, Tryptophan, Isoleucine, Methionine, Histidine, Leucine and Lysine. You can remember them with this mnemonic PVT. TIM HALL.
Ø Please note, there are NO essential amino acids in plants and bacteria, they can synthesize all amino acids.
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
(2). Conditionally Essential Amino acids
Ø Conditionally essential amino acids are those amino acids whose synthesis can be limited under special pathophysiological or developmental conditions.
Ø Examples: Arginine, Cysteine, Glycine, Glutamine, Proline and Tyrosine
(3). Non-essential Amino Acids
Ø Our body can synthesize these amino acids from precursor molecules.
Ø Examples: Alanine, Aspartic Acid, Asparagine, Glutamic Acid, Serine and Selenocysteine and Pyrolysine (considered the 21st * 22nd amino acids)
(F). Classification based on Metabolic Fate
Ø This classification is based on the fate of amino acids during their catabolism.
Ø Based on metabolic fate, amino acids can be of TWO types:
(1). Glucogenic Amino Acids
(2). Ketogenic Amino Acids
(1). Glucogenic Amino Acids:
o Glucogenic amino acids are converted into glucose through gluconeogenesis.
o They are converted into pyruvate, α-ketoglutarate, succinyl CoA, fumarate, or oxaloacetate.
o Examples: arginine, glutamate, glutamine, histidine, proline, valine, methionine, aspartate, asparagine, alanine, serine, cysteine, and glycine
(2). Ketogenic Amino Acids:
Ø Ketogenic amino acids are converted into ketone bodies during catabolism.
Ø They are converted into acetyl Co-A or acetoacetate.
Ø Examples: Lysine and leucine
Ø Note: Some amino acids are both glucogenic and ketogenic. Example: tryptophan, phenylalanine, tyrosine, isoleucine, and threonine
References
Lehninger A.B., (2018), Textbook of Biochemistry, Ed. 5, Pearson International, New York
<<< Back to BIOCHEMISTRY Notes Page
More Lecture Notes from Easy Biology Class…
BotanyZoologyBiochemistryGeneticsCell & Molecular BiologyBiotechnologyPhysiology & EndocrinologyPlant PhysiologyMicrobiologyImmunologyEmbryologyEcologyEvolutionBiophysicsResearch MethodologyBiostatisticsChemistry for BiologistsPhysics for Biologists