Enzymes are biological catalysts that play a pivotal role in facilitating various biochemical reactions within living organisms. The study of enzyme kinetics is essential to understand the dynamics of enzymatic reactions. One of the critical parameters in enzyme kinetics is the Michaelis-Menten constant (Km). This post will help you to understand What is Km in Enzyme Kinetics.
| Biochemistry Notes | Biochemistry PPT | Biochemistry MCQs |
Enzyme Kinetics and Michaelis-Menten Equation
Ø Enzyme kinetics explains the rates at which enzymatic reactions occur.
Ø It also describes the factors that influence the rate of enzymatic reactions.
Ø The Michaelis-Menten equation provides a fundamental understanding to the kinetics of enzyme-substrate interactions.
Ø It also offers insights into the essential properties of enzymatic reactions.
Ø Michaelis-Menten equation is represented as V0 = (Vmax * [S]) / (Km + [S]).
Ø Where.
V0 is the reaction velocity (initial velocity)
[S] is the substrate concentration
Vmax is the maximum velocity of the reaction
Km is the Michaelis-Menten constant
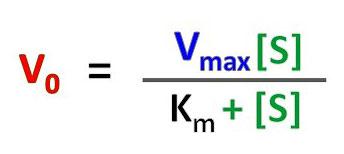
What is Km in Enzyme Kinetics?
Ø Km is Michaelis-Menten constant.
Ø Definition: Km represents the substrate concentration at which an enzyme operates at half of its maximum velocity (Vmax).
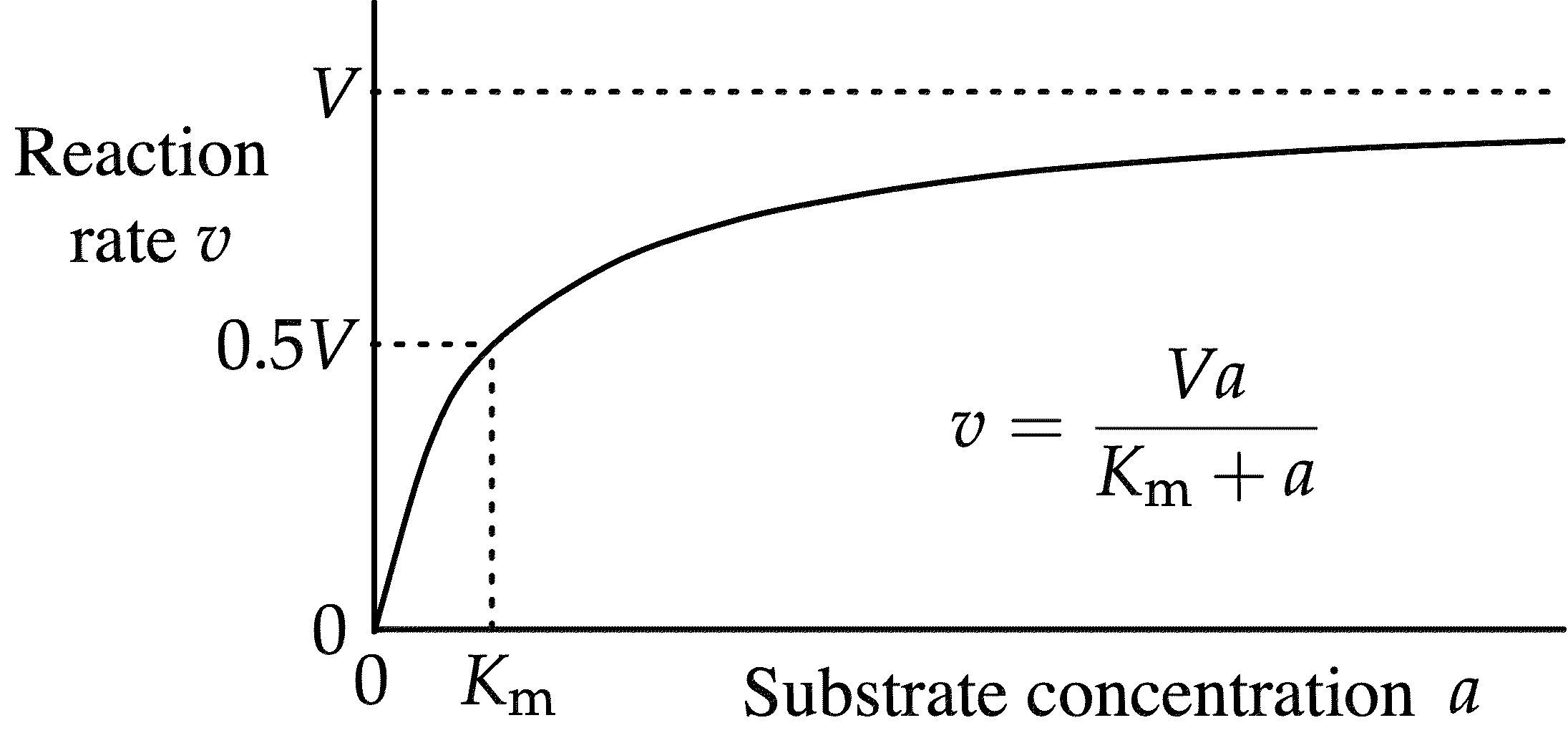
Ø Km represents the affinity of the enzyme for its substrate.
Ø Low Km values indicate high affinity, suggesting that the enzyme effectively binds to the substrate even at low concentrations.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Ø High Km values suggest lower affinity and a need for higher substrate concentrations for effective binding.
Ø Thus, Km forms the basis for analysing the relationship between substrate concentration and reaction velocity.
Ø Understanding Km has immense significance in elucidating the behaviour of enzymes and their catalytic efficiency.
Significance of Km
(1). Affinity of Enzymes for Substrates
Ø Km serves as a quantitative measure of the enzyme’s affinity for its substrate.
Ø Enzymes with low Km values exhibit strong affinity, as they can effectively convert substrates into products even at low concentrations.
Ø Enzymes involved in essential biochemical pathways having an optimal Km ensures that reactions proceed efficiently.
(2). Determining Catalytic Efficiency
Ø The relationship between Km and Vmax plays a vital role in determining the catalytic efficiency of an enzyme.
Ø Enzymes with both high Vmax and low Km are more efficient.
Ø These enzymes can convert substrates to products rapidly and effectively.
(3). Insight into Enzyme Specificity
Ø Km can provide insights into enzyme specificity.
Ø Enzymes with low Km values are highly specific to their substrates.
Ø Enzymes with higher Km values might have broader substrate specificity.
Learn more: Specificity of Enzymes Notes
(4). Drug Design and Enzyme Inhibition
Ø Understanding Km very important in drug design and understanding enzyme inhibition.
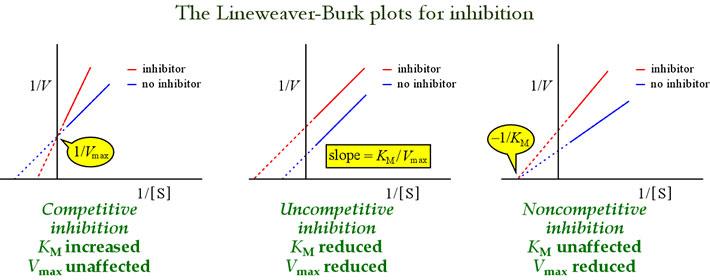
Ø Inhibition of enzymes often involves manipulating Km values.
Ø Example: Competitive inhibitors raise the apparent Km, leading to a decrease in enzyme-substrate affinity.
Ø Non-competitive inhibitors alter the Vmax leaving the Km unchanged.
(5). Optimization of Biotechnological Processes
Ø In biotechnology and genetic engineering, understanding Km values aids in optimizing enzymatic reactions.
Ø By determining the appropriate substrate concentrations, reaction conditions can be fine-tuned to achieve maximal product yields.
Ø This makes enzyme-mediated processes more efficient and economically viable.
Learn more: Enzyme Immobilization
Summary
Michaelis-Menten constant, Km, is a vital parameter in enzyme kinetics that sheds light on the substrate affinity, catalytic efficiency, and specificity of enzymes. Its significance spans from fundamental biochemical understanding to practical applications in drug design, biotechnology, and enzyme-related processes.
<<< Back to Biochemistry Notes Page
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
