Covalent vs Hydrogen Bond
Difference between Covalent and Hydrogen Bond: The chemical bonds are stable attractions between atoms, ions or molecules. The formation of chemical bonds allows the formation of molecules or compounds. Chemical bonds are classified into different categories based on their formation and strength. They are categorized as Covalent bonds, Ionic bonds, Metallic bonds, Dipole-dipole interactions, London dispersion forces and Hydrogen bonds.
Learn more: Formation of Hydrogen Bond in Water
The present post discusses about the Differences between the Covalent bond and Hydrogen bond with a Comparison Table.
Covalent bond is a primary chemical bond formed by the sharing of electron pairs. Covalent bonds are strong bonds with greater bond energy.
You may also like NOTES in...
BOTANY BIOCHEMISTRY MOL. BIOLOGY
ZOOLOGY MICROBIOLOGY BIOSTATISTICS
ECOLOGY IMMUNOLOGY BIOTECHNOLOGY
GENETICS EMBRYOLOGY PHYSIOLOGY
EVOLUTION BIOPHYSICS BIOINFORMATICS
Hydrogen bond is a weak electrostatic attraction between the hydrogen and an electronegative atom due to their difference in electronegativity. Individual hydrogen bonds are weak bonds however, their presence in large number provide them considerable strength.
Learn more: Covalent Bond vs Ionic Bond
Learn more: Covalent Bond vs Metallic Bond
Difference between Covalent and Hydrogen Bond
Sl. No. Covalent Bond Hydrogen Bond
1 Covalent bond is formed by the mutual sharing of electrons between atoms Hydrogen bond is formed by the electrostatic attraction between hydrogen and an electronegative atom such as O, N and F
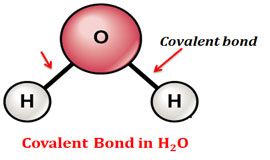
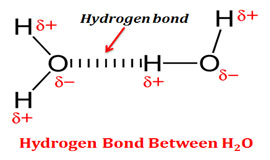
2 Example: Bond between hydrogen and oxygen in the water molecule (H – O – H) Example: Interaction between water molecules in liquid water and ice, the interaction between the two strands of DNA
3 The covalent bond is a strong bond Hydrogen bond is a weak bond
4 The bond energy of covalent bond is between 100 to 1100 kJ/mol Bond energy of hydrogen bond is between 5 to 50 kJ/mol
5 Covalent bond is a primary bond Hydrogen bond is a secondary bond
6 Covalent bond changes the chemical properties of the bonding constituents Hydrogen bond changes the physical properties of bonding constituents
7 Covalent bonds can be formed between any atoms with suitable electron valency Hydrogen bonds can occur only between hydrogen and an electronegative atom
8 Covalent bond formation ensures to fulfill the valency of the outer orbitals in an atom Hydrogen bond formation will not fulfill the valency of atomic orbitals
9 Covalent bonds can occur between polar and non-polar atoms or molecules Hydrogen bonds can occur only in polar molecules
10 Covalency will be greatest if the bonded atoms have similar electronegativity Strength of hydrogen bond increases with increase in the electronegativity difference between hydrogen and the bonded atom
11 Covenant bonds will always be intra-molecular bonds Hydrogen bonds will always be inter-molecular (never intra-molecular)
