Learning Objectives- Amino Acids: Structure and Functions: What are Amino Acids? List of Amino Acids, Three Letter Code and Single Letter Code of Amino Acids, Discovery of Amino Acids, Structure of Amino Acids, Proteogenic Amino Acids and Non Proteogenic Amino Acids, Alpha Amino Acids and Beta Amino Acids, Isomerism in Amino Acids, Zwitterion, Isoelectric pH of Amino Acids, Functions of Amino Acids.
What are Amino Acids?
Ø Amino acids are organic compounds that contain both amino group and carboxylic groups.
Ø They are the building blocks of protein. Proteins are the polymers of amino acids.
Ø During protein synthesis (Transcription) amino acids are joined together by a special type of covalent bond called the peptide bond. Proteins on hydrolysis yield amino acids.
Ø There are 22 different amino acids (list given below) found in protein, or we can say all proteins or the life domain are the combinations of these 22 amino acids. Hence, they are called ‘Magic-22’.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
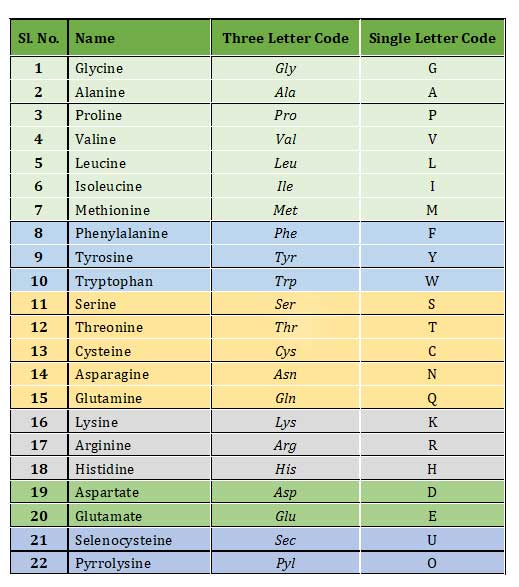
List of Amino Acids and their Three Letter and Single Letter Code
NB: You are advised to memorize both the three-letter code and single letter code of each amino acids. It is very essential to know the single letter code of all amino acids since the sequence of proteins will be represented in single letter code in biochemistry, molecular biology and bioinformatics.
Discovery of Amino Acids
Ø The first discovered amino acid was Asparagine from Asparagus in 1806. Cystine was discovered in 1810, however, its monomer, cysteine, remained undiscovered until 1884.
Ø Glutamate was first isolated from wheat gluten. Tyrosine was first isolated from Cheese and hence the name.
Ø Glycine and leucine were discovered in 1820. Glycine is the simplest amino acid and the name glycine is due to its sweet taste.
Ø The last proteogenic amino acid discovered was Pyrrolysine in 2002.
Proteogenic Amino Acids
Ø More than 500 naturally occurring amino acids are known now. However, only 22 (listed in the above table) appear in proteins and they are called Proteinogenic Amino Acids.
Ø Proteinogenic Amino Acids: Amino acids that are incorporated biosynthetically into proteins during translation. Proteogenic amino acids will have codons and tRNA.
Ø There are 20 + 2 (22) genetically encoded proteinogenic amino acids. Among these, the first 20 amino acids (as given in the table) are coded by the standard genetic code.
Ø The remaining two 2 amino acids Selenocysteine (21st amino acid) and Pyrrolysine (22nd amino acid) are coded by stop codons in a special translation mechanism.
Ø Selenocysteine is coded by UGA codon (opal stop codon) and Pyrrolysine is coded by UAG codon (amber stop codon)
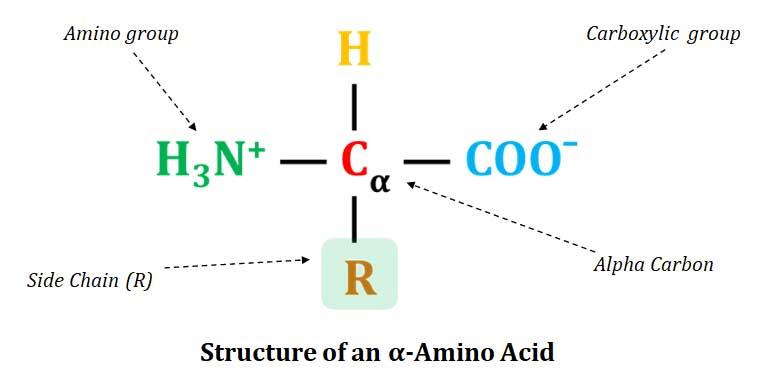
Structure of Amino Acids (Proteinogenic Amino Acids)
Ø Amino acids are organic compounds.
Ø All proteinogenic amino acids contain a central carbon called the α-carbon.
Ø The FOUR valances of the α-carbon atom are satisfied by:
§ Amino group (-NH2)
§ Carboxylic group (-COOH)
§ Hydrogen (H)
§ Side chain (R – Group)
Ø The side chain (R) is specific to each amino acid.
Ø The key elements of an amino acid are C, H, O and N.
Ø Other elements are found in the side chains of certain amino acids: Sulphur (S) as in Cysteine and Methionine; Selenium (Se) as in Selenoysteine.
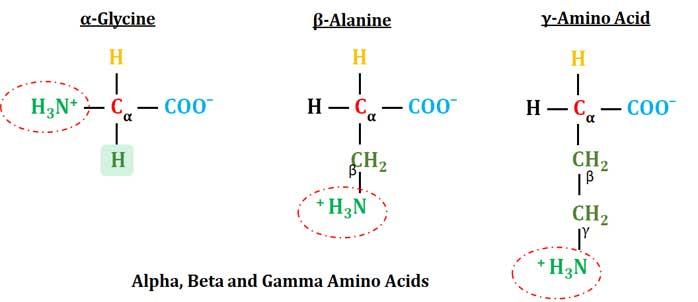
All Proteogenic Amino Acids are α-Amino Acids
Ø All protein-coding amino acids are α-amino acids.
Ø α-Amino Acid: Amino acids containing an amino group (primary amino group) bonded directly to the α-carbon atom is called α-amino acid.
Ø In some amino acids, the amine group is attached to the β or γ-carbon, and these are therefore referred to as beta or gamma amino acids respectively.
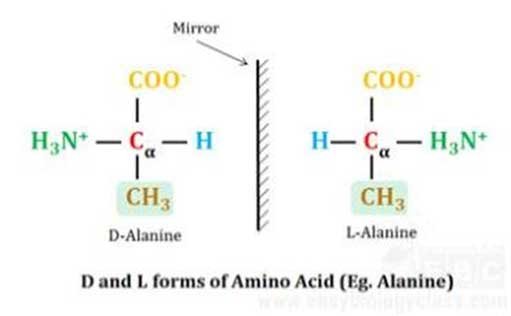
Isomerism in Amino Acids
Ø The α-carbon atom in all amino acids (except in glycine) is asymmetric (chiral). i.e., the four valences are satisfied by four different groups (-NH2, -COOH, -H and -R). In the case of Glycine, the side chain R is -H, thus glycine has a symmetric α-carbon atom.
Ø Due to the presence of asymmetric carbon, the amino acids can form TWO stereo-isomers called enantiomers. We have discussed the concept of enantiomers in Carbohydrates. Please read that as well.
Ø These enantiomers are named D-amino acid and L-amino acid.
Ø All usual protein-coding amino acids are L-isomers. (Please remember, all usual monosaccharides are D isomers).
Ø D-amino acids are rarely found in nature.
Ø D-amino amino acids naturally occur in peptidoglycan. In peptidoglycan, the short peptide cross-links that connects parallel NAM and NAG chain consist of D- and L- amino acids.
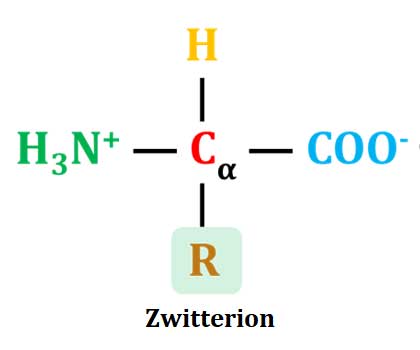
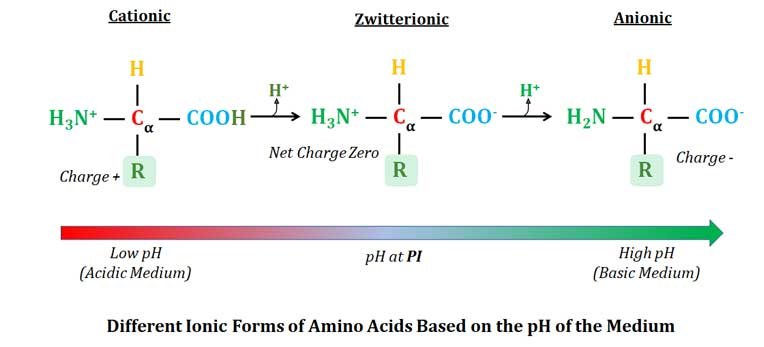
Zwitterions
Ø Based on the pH of the medium, an amino acid can exist in THREE different ionic forms, they are:
§ Cation (Positively charged ion) at low pH (Fully protonated form).
§ Zwitterion (Net charge zero) at pI
§ Anion (Negatively charged ion) at high pH (Fully deprotonated form)
NB: When the pH is below the pI the amino acid will be cationic; when the pH is above pI the amino acid will be anionic; When the pH is at pI the amino acid will be Zwitterionic
Ø All amino acids are amphiprotic in the aqueous medium.
Ø Amphiprotic: A molecule or ion that can act as both acid (donate proton) and as base (accept proton) is called amphiprotic.
Ø The ionic form of amino acid with one positive and one negative charge is called Zwitterion. The net charge of zwitterion will be zero (equal number of positive and negative charges).
Ø Zwitterion: A molecule with two functional groups, of which one has a positive and the other has a negative electrical charge and the net charge of the entire molecule is zero is called Zwitterion.
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
What is Isoelectric Point (IEP)?
Ø The isoelectric point is also called Isoelectric pH.
Ø It is denoted as pI or IEP
Ø Definition: Isoelectric point is the pH at which a molecule carries NO net charge (equal number of positive and negative charges). i.e., the molecule is in the form of a zwitterion.
Ø At pI, the molecule DO NOT migrate towards anode or cathode even though they contain charged ends.
Ø More importantly, at pI, an amino acid can act as both acid (proton donor) and a base (proton acceptor).
Ø The protonated amino group (-NH3+) can donate proton whereas the deprotonated carboxylic group (-COO–) can accept protons.
Ø Amino acids form zwitterions at their isoelectric point.
Ø The isoelectric point is the principle behind a separation technique called Iso-Electric Focusing.
Ø Iso-electric focusing is used for the purification of proteins and amino acids from crude mixtures using electrophoresis.
Functions of Amino Acids
Ø Amino acids are the building blocks of proteins. Amino acids joined together according to the sequence of the codon in the in the mRNA to form the specific proteins in the cell and thereby they participate in the expression of genes.
Ø Apart from their role as building blocks of proteins, amino acids also perform some other functions.
Ø Many amino acids act as the precursors for the biosynthesis of other important metabolites and metabolic intermediates. Some of them are listed below.
Ø Glutamate, the anionic form of glutamic acid is the principal excitatory neurotransmitter
Ø Gamma-aminobutyric acid (GABA), the inhibitory neurotransmitter is synthesized from glutamate.
Ø Tryptophan is a precursor of serotonin (a neurotransmitter) in animals.
Ø Tryptophan is also the precursor of natural auxin (indol-3-acetic acid) in plants.
Ø Tyrosine and Phenylalanine are the precursors of the catecholamine neurotransmitters dopamine, epinephrine and norepinephrine.
Ø Phenylalanine is a precursor of tyrosine in humans
Ø Phenylalanine is the precursor of various phenylpropanoids in plants.
Ø Glycine is the precursor of porphyrins such as heme.
Ø Arginine is a precursor of nitric oxide.
Ø Ornithine is the precursor of polyamines
Ø Aspartate, glycine, and glutamine participate in the biosynthesis of nucleotides.
Notes on Nucleotide Biosynthesis
<<< Back to Biochemistry Notes Page
You may also like: | Protein Structure | Bonds in Proteins | Classification of Proteins | Properties of Enzymes |
More Lecture Notes from Easy Biology Class…
Botany|Zoology|Biochemistry|Genetics|Cell & Molecular Biology|Biotechnology|Physiology & Endocrinology|Plant Physiology|Microbiology|Immunology|Embryology|Ecology|Evolution|Biophysics|Research Methodology|Biostatistics|Chemistry for Biologists|Physics for Biologists
Browse more in Easy Biology Class…
Lecture Notes|Biology PPTs|Video Tutorials|Biology MCQ|Question Bank|Difference between|Practical Aids|Mock Tests (Online)|Biology Exams

Good day, how can I upload pdf version of this document? thank you for your help, you’re the best website for science students.
For some notes you can get the pdf version below the post content.
Very soon you will be able to download all the content, we are working on int
Keep visiting easybiologyclass
Regards