What are Enzymes?
There are thousands of chemical reactions in a living system. The chemical reactions in the cell are catalyzed by the biological catalysts called enzymes. Almost all enzymes are highly specialized proteins. (Exception: Ribozymes –Ribozymes are RNA with catalytic activity). The current post we will discuss the Characteristics of Enzymes. We will also discuss the features of a Catalyst and the concept of Activation Energy of a reaction.
What are catalysts?
Ø The catalyst is substances that accelerate the rate of a chemical reaction.
Ø The catalyst is not consumed or transformed by the reaction.
Ø It will not change the equilibrium constant of the reaction.
Ø Catalysts only change the rate to approach equilibrium constant.
Ø Catalysts are not required in stoichiometric quantities.
Ø Examples: Platinum, Palladium etc.
Brief History about Enzymes
 Ø The history of biochemistry is the history of enzyme research.
Ø The history of biochemistry is the history of enzyme research.
Ø Louis Pasteur reported fermentation of sugar into alcohol by yeast is catalyzed by “ferments”.
Ø Frederick W. Kuhne coined the term ENZYME for the ‘ferments’.
Ø The first enzyme discovered was Diastase from malt by Anselme Payen in 1833.
Ø The first crystallized enzyme is Urease by James Sumner.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Characteristics of Enzymes
Ø The enzymes have extraordinary catalytic power.
Ø Enzymes accelerate reactions up to 1014 to 1020 times.
Ø Enzymes have a high degree of specificity for their substrates and reactions.
Ø They function in an aqueous solution.
Ø Enzymes work under a mild condition of temperature and pH.
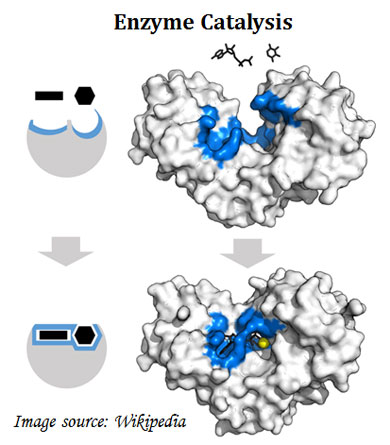
Ø Enzymes make macromolecules from simple precursors.
Ø The enzymes act in an organized sequence.
Ø They catalyze the hundreds of step-wise reaction.
Ø Enzymes can regulate metabolic pathways and these enzymes are regulatory enzymes.
Ø In some genetic disorders, there may be a deficiency one or several enzymes (Eg. albinism).
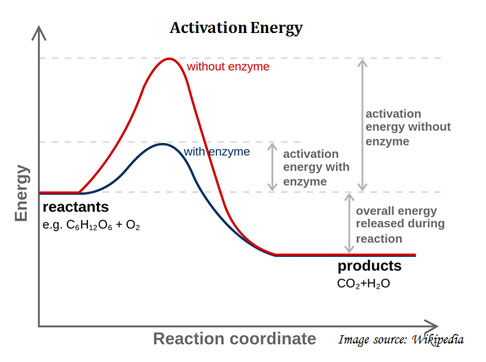
Ø Enzyme reduces the activation energy of the reaction.
Activation Energy
Ø The term activation energy was introduced by Svante Arrhenius (1889).
Ø Definition: “The minimum energy that must be input to a chemical system, containing potential reactants, in order for a chemical reaction to occur”.
Ø In simple term, the minimum energy required to start a chemical reaction.
Ø For a chemical reaction to proceed at a reasonable rate, there should exist an appreciable number of molecules with energy equal to or greater than the activation energy.
Ø The activation energy of a reaction is denoted as Ea.
Ø The Ea is given in units of kilo-joules per mole.
Enzymes Structure
Ø All enzymes are proteins except Ribozymes. Ribozymes are specialized RNA molecules with catalytic activity.
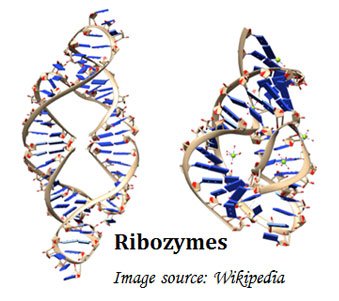
Ø The catalytic activity of an enzyme depends on the integrity of the enzyme’s native conformation.
Ø The primary, secondary, tertiary & quaternary structures of protein are essential for its catalytic properties.
Learn more: Protein Structure (Primary, Secondary, Tertiary & Quaternary)
Ø The denatured enzyme will not have catalytic activity.
Ø Most of the enzymes consist of multi-subunits (more than one polypeptide chains).
Ø Some enzymes require no chemical groups for activity other than their amino acid residues.
Ø Others enzymes require additional chemical components (one or more) for their activity.
Ø Enzymes are much larger than their substrates.
Ø The smallest enzyme 4-oxalocrotonate tautomerase consists of 62 amino acid residues.
Ø The largest enzyme Fatty acid synthase consists of ~ 2000 amino acid residues.
Ø Even though most of the enzymes contain thousands of amino acids only 2–4 amino acids are directly involved in the catalysis.
Ø Binding Sites in the enzyme:
Substrate binding site: the areas of an enzyme where the substrate binding occurs.
Catalytic site: one or many sites, located near to the binding site. They perform the catalysis.
Active site: Binding site and catalytic site together called active site.
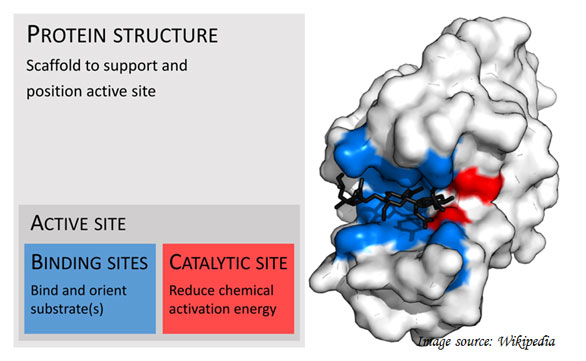
Cofactor site: Additional sites for the binding of cofactors.
Allosteric site: Additional sites for the binding of allosteric modulators. Allosteric modulators are involved in the regulation of enzymatic activity.
Learn more: Regulatory Enzymes
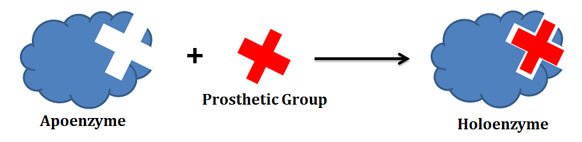
Apoenzyme and Holoenzyme
Ø Apoenzyme (apoprotein): The protein part of an enzyme is called apoenzyme.
Ø Prosthetic group: The non-protein part of an enzyme is called the prosthetic group.
Ø Holoenzyme: The fully functional apoenzyme and the required prosthetic group together are called holoenzyme.
Ø Holoenzyme = Apoenzyme + Prosthetic Group
Cofactors and Coenzymes
Ø The prosthetic groups of an enzyme are of different types and they are broadly categorized into two groups.
(1). Cofactors
(2). Coenzymes
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
Cofactors
Ø Cofactors: A non-protein chemical compound in an enzyme that is bound to an enzyme is called the cofactor.
Ø They are tightly bound to the enzyme.
Ø Cofactors may be organic groups or inorganic groups.
Ø Inorganic cofactors include metal ions such as Fe2+, Mg2+, Mn2+, Zn2+ and iron-sulfur clusters.
Ø Organic cofactor includes Flavin and Haem.
Ø Cofactors are required for the proper functioning of enzymes.
Ø Some enzymes require several cofactors.
Ø Example: The pyruvate dehydrogenase of the link reaction of respiration requires five cofactors. They are:
(1). Metal ion
(2). Loosely bound thiamine pyrophosphate (TPP)
(3). Covalently bound lipoamide
(4). Flavin adenine dinucleotide (FAD)
(5). Co-substrates (NAD, Coenzyme-A and Mg2+)
Coenzymes
Ø Co-enzyme: Additional chemical component in the enzyme (prosthetic group) which is complex organic or metallo-organic molecules.
Ø The main difference from cofactor is that coenzymes are NOT tightly bound to the enzyme.
 Ø Coenzymes act as the carriers of specific functional groups.
Ø Coenzymes act as the carriers of specific functional groups.
Ø They transport chemical groups from one enzyme to another
Ø Most of the coenzymes are derived from vitamins.
Ø Co-enzyme is released from the enzyme’s active site during the reaction.
Ø Usually, coenzymes are chemically modified after the catalytic reaction.
Ø Thus, the coenzymes are considered as the second substrate.
Ø Examples for co-enzymes: NADHH+, NADPHH+, ATP
Ø One coenzyme is common to many different enzymes.
Ø Example: The NADHH+ is a coenzyme for about 700 different enzymes in human.
Ø Coenzymes are continuously generated in the cell.
Ø Their concentration is maintained at a steady level in the cell.
<<< Back to BIOCHEMISTRY Home Page
You might also like…
@. Enzyme Regulation Mechanism
@. Enzyme Substrate Specificity
@. Proteins: Structure and Functions

If pdf download is not available for total enzyme chapter of biochemistry ❓
The lecture note on the structure and characteristics of enzymes is comprehensive, simplified and very thorough. I am very grateful and happy to be able to access this site free of charge. I really commend the scientists who panistakingly put this valuable lecture note together. My thanks to the authors of this immensely valuable work. Hope u will keep up this good work. Isaac Osagie
Hii !
I am really glad to find this site this is amazing and the explanation is eazy understandable i am really happy more over it is free to access and user friendly and i hope you post more in future
Thanks
Thank you Archana
Keep visiting easybiologyclass