What are Sugar Derivatives?
Organisms contain a variety of sugar derivatives. Sugar derivatives are modified monosaccharides. In sugar derivatives, the monosaccharide molecules that have been modified with substituents other than hydroxyl groups such as amino groups, acid groups, phosphate groups, acetate groups etc. Natural sugar derivatives have important biological functions. Majority of sugar derivatives are hexose derivatives. In this post we discuss about sugar derivatives with examples and their functions in the cells.
Table of Contents
$. Examples of Sugar Derivatives
@. Aldonic Acids
@. Uronic Acids
@. Aldaric Acids
@. Alditols
@. Deoxy-sugars
@. Amino-sugars
@. Sialic Acids
Examples of Sugar Derivatives
Ø There are TWO main modes of formation of sugar derivatives:
(1). Hydroxyl group in the sugar is replaced with another substituent.
(2). Oxidation of carbon in the chain to form carboxyl group.
Ø The following are the important classes of sugar (monosaccharide) derivatives:
(1). Aldonic Acids
(2). Uronic Acids
(3). Aldaric Acids
(4). Alditols
(5). Deoxy-sugars
(6). Amino-sugars
(7). Sialic Acids
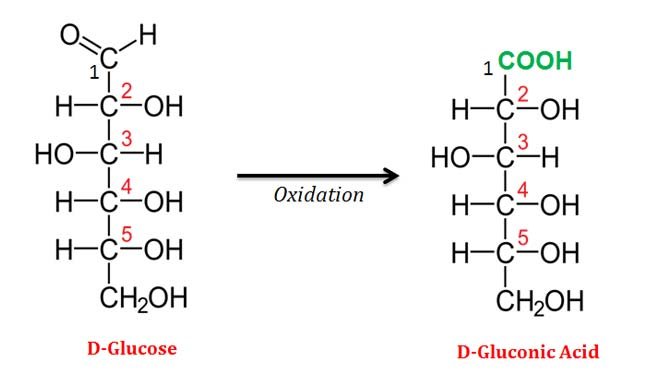
(1). Aldonic Acids
Ø Aldonic acids are formed by the oxidation of an aldose (at C1) converts its aldehyde group to a carboxylic acid group.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Ø Aldonic acids are named by appending the suffix -onic acid to the root name of the parent aldose (E.g.: Aldonic acid of glucose is named as Gluconic Acid).
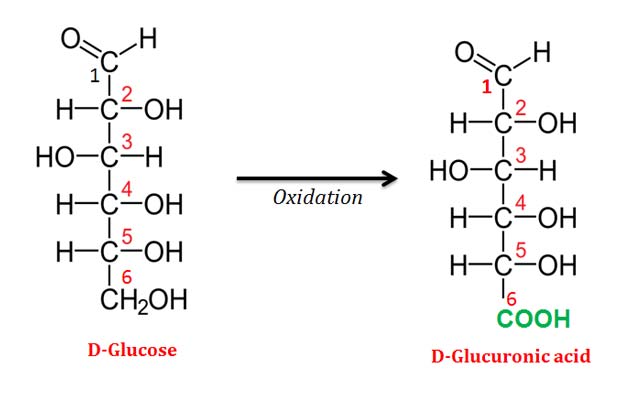
(2). Uronic Acids
Ø Uronic acids are formed by the oxidation of the primary alcohol group (the – OH group at C-6 position) of an aldose sugar.
Ø Uronic acids are named by appending -uronic acid to the root name of the parent aldose.
Ø Example: Uronic acid of D-Glucose is named as D-Glucuronic acid.
Ø Uronic acids can assume the pyranose, furanose, and linear forms.
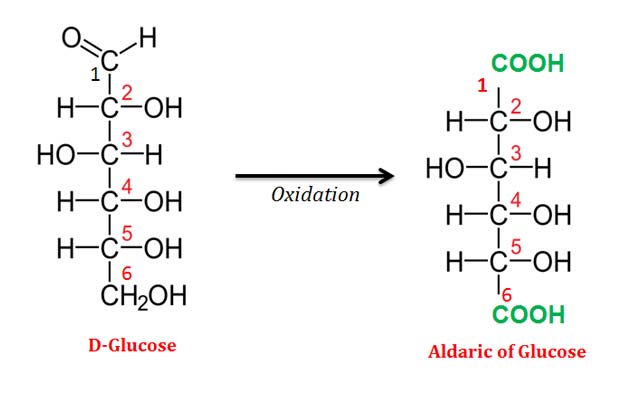
(3). Aldaric Acids
Ø In aldaric acids, the terminal carbonyl groups (at C1) and terminal hydroxyl group (at C6 in the case of hexose) have been replaced by terminal carboxylic acids.
Ø Aldaric acids have the general chemical formula HOOC-(CHOH)n-COOH.
Ø Aldaric acids do not form cyclic structures.
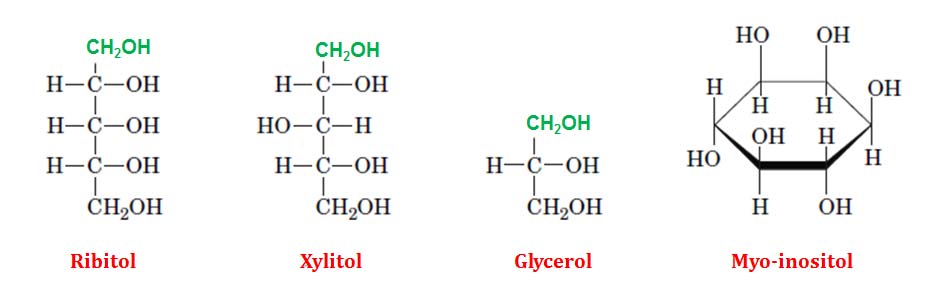
(4). Alditols
Ø Alditols are polyhydroxy alcohols.
Ø Alditols are formed by the reduction of aldoses by mild reducing agents to yield polyhydroxy alcohols.
Ø Alditols are named by appending the suffix -itol to the parent aldose.
Ø Example: Ribitol, Glycerol, Xylitol, Myo-inositol.
Ø Glycerol and myo-inositol (cyclic polyhydroxy alcohol) are important components of lipids.
Ø Xylitol is a sweetener that is used in “sugarless” candies.
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
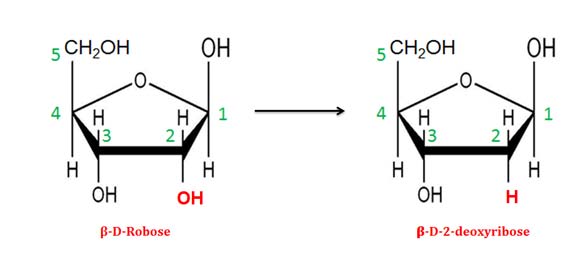
(5). Deoxy sugars
Ø Monosaccharides in which an -OH group is replaced by –H is called doxy sugars.
Ø Example: 𝛃-D-2-deoxy ribose.
Ø Deoxy sugar of ribose is the sugar component of DNA.
Ø L-Fucose (6-deoxy-l-galactose): Deoxy sugar of L-Galactose.
Ø L-Fucose is one of the few L sugar components of polysaccharides (fucoidan).
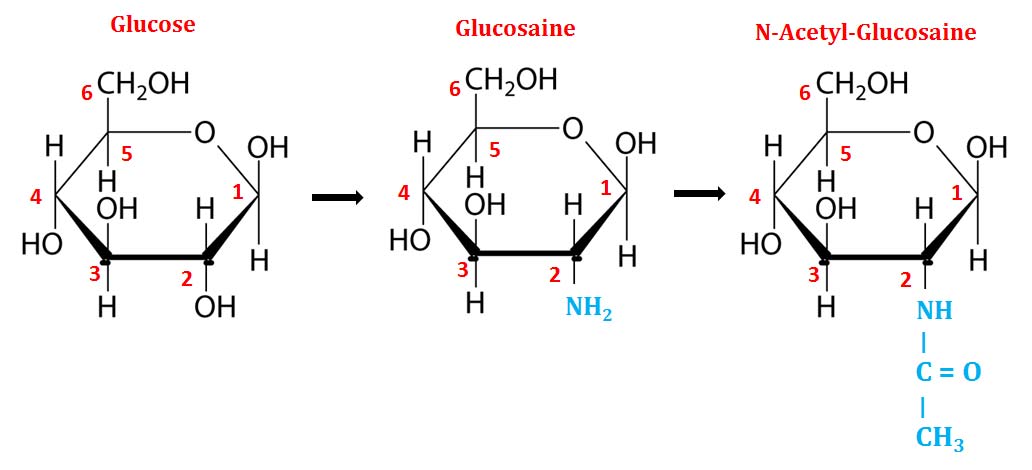
(6). Amino-Sugars
Ø In amino sugars, the -OH group at C2 is replaced by an amino (-NH2) group.
Ø The attached amino group is often acetylated, and are named as N-acetyl-.
Ø Example: D-Glucosamine, D-Galactosamine and D-Mannosamine.
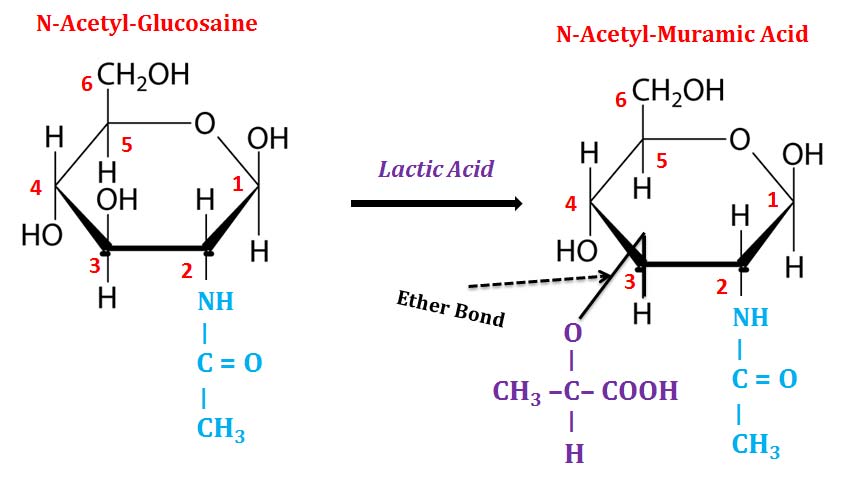
Ø Lactic acid ether (at C3) of glucosamine is called muramic acid.
Ø Acetylated muramic acid is called N-Acetyl-muramic acid.
Ø N-Acetyl-Glucosamine (NAG) and N-Acetyl-Muramic acid (NAM) are the building blocks of peptidoglycan (cell wall of bacteria).
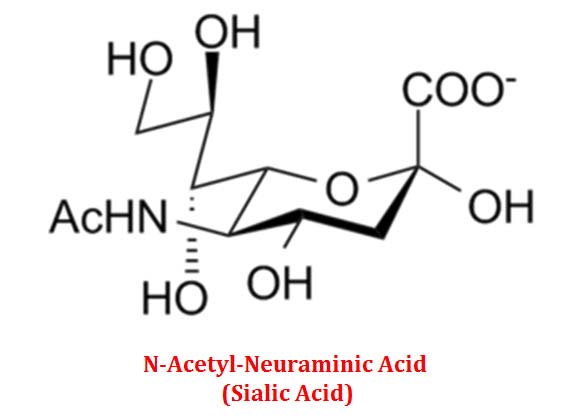
(7). Sialic Acids
Ø N-acetylmannosamine and Pyruvic Acid combine to form N-Acetylneuraminic acid (NANA).
Ø NANA has a backbone of 9 carbons (6+3).
Ø NANA and its further derivatives are collectively called as Sialic acids.
Ø Sialic acids are important sugar components in glycoproteins and glycolipids.
References
@. Lehninger A.B., (2018), Textbook of Biochemistry, Ed. 5, Pearson International, New York
@. Voet, D., Voet, J.G. and Pratt, C.W., 2013. Fundamentals of biochemistry: life at the molecular level
Do you have any Queries? Please leave me in the Comments Section below.
I will be Happy to Read your Comments and Reply.
<<< Back to Biochemistry Notes Page
You may also like…
Introduction to Carbohydrates | Monosaccharides | Disaccharides | Polysaccharides | Glycosaminoglycans | Sugar Derivatives | Glycoconjugates |

Wooow the lecture is so amazing 🤩
Thank you
its really helpful website for learning and its easy to understand I like this website
this is very helpful website for learning excellent way of disscusion and easy to understand