Proton Hopping in water is the process of diffusion of protons (H⁺ ions) through the network of hydrogen-bonded water molecules in the liquid water. Proton hopping is also called as Grotthuss mechanism, named after the discoverer Theodor Grotthuss. The net result of proton hopping is the fast movement of H⁺ ions in water than any other dissolved cations such as Na⁺ or K⁺. Let’s see how proton hopping occurs in water.
Water has a slight tendency of ionization. The H2O molecules can ionize into H⁺ and OH¯ ions as in the equation (1).
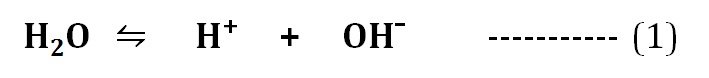
The ionization reaction of water can be described by its equilibrium constant. The equilibrium constant (Keq) of the ionization of water at 25°C is calculated as 1.8 X 10¯16 M. The equilibrium constant and the concentration of H⁺ and OH¯ ions formed as a result of the ionization of H2O molecules are responsible for the pH of water.
Learn more: Why the pH of Water is 7?
Even though the water ionizes to produce H⁺ and OH¯ ions (equation 1), the free H⁺ ions do not occur in the water. The H⁺ ions formed will immediately bind to an intact water molecule to form Hydronium ions (H3O⁺) as in the equation (2).
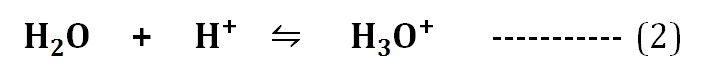
The frequent occurrence of hydrogen bonds in water allows the quick formation of hydronium ions as given in the equation (3).
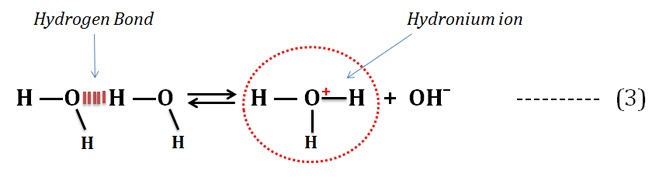
Learn more: How Hydrogen Bond is formed in Water?
The degree of ionization of water can be determined by its electrical conductivity. Pure water can carry electrical current as the H⁺ ions migrate towards the cathode and the OH¯ ions move towards the anode. The movement of H⁺ ions (also hydronium ions) and OH¯ ions under the electric field is relatively faster than other cations and anions such as Na⁺, K⁺ and Cl¯. The rapid movement of H⁺ and OH¯ ions in water is achieved by the proton hopping mechanism.
You may also like NOTES in...
BOTANY BIOCHEMISTRY MOL. BIOLOGY
ZOOLOGY MICROBIOLOGY BIOSTATISTICS
ECOLOGY IMMUNOLOGY BIOTECHNOLOGY
GENETICS EMBRYOLOGY PHYSIOLOGY
EVOLUTION BIOPHYSICS BIOINFORMATICS
The process of proton hopping is summarized in the following image.
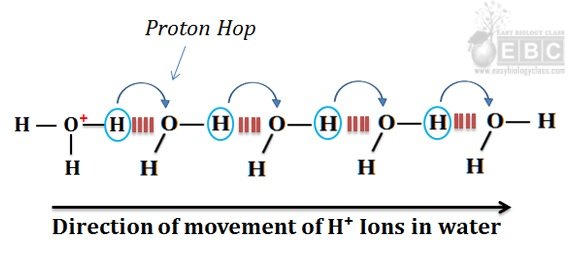
Proton hopping (hops) between a series of hydrogen-bonded water molecules cause rapid net movement of H⁺ or OH¯ ions over a long distance. (Caption for the image)
In the figure, it is clear that when a hydronium ion releases the H⁺ ion, a nearby water molecule accepts it and form another hydronium ion. This process is continued very quickly due to the driving force of the electric current. The net effect of proton hopping is the quick and rapid movement of H⁺ ions towards the cathode. This movement of H⁺ ions is comparatively faster than any dissolved cations (such as Na⁺ or K⁺) in water. The migration of Na⁺ and K⁺ ions in the water towards the cathode is achieved by diffusion through the water molecules, whereas the movement of H⁺ ions towards the cathode is due to proton hopping. The proton hopping is comparatively faster than diffusion. The HO¯ ions can also ‘hop’ in water and move the same speed as H⁺ ions. However, the direction of movement will be opposite to that of H⁺ ions (towards the anode).
Significance of proton hopping in the biological system:
Proton hopping plays a crucial role in the biological proton transfer reactions such as the movement of H⁺ ions in the cellular or organellar matrix.
References
Lehninger A.B., (2018), Textbook of Biochemistry, Ed. 5, Pearson International, New York
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
You might also like…
@. How Hydrogen Bond is formed in Water?
@. Ion Product and pH of Water
@. Chemical and Physical Properties of Water
@. Deriving Henderson-Hasselbalch Equation
@. How to calculate pH and pKa of a Buffer
