Physical and Chemical Properties of Water: Water is the most abundant substance in the living system. Water makes up about 70% or more of the weight of almost all organisms. The life has originated in remote past in the aqueous environment. The properties (both physical and chemical) of water enabled it as the ‘solvent of life’. The water possesses some unusual physical and chemical properties. These ‘unusual properties’ are responsible making water as the ‘solvent of life’.
The present post describes the Physical, Chemical and Unusual Properties of Water. We will also discuss the importance or significance of these properties of water in the biological system.
The unusual properties of water are mainly due to three factors:
(A). The small size of water molecules
(B). The polarity of water molecules
(C). The formation of hydrogen bonds between adjacent water molecules
Most important physio-chemical and unusual properties of water can be summarized into the following heads:
(1). Solvent properties of water
(2). High heat capacity of water
(3). High heat of vaporization
(4). High heat of fusion
(5). Density and freezing properties
(6). High cohesion, adhesion and surface tension of water
(7). Water acts as a reactant
(1). Solvent properties of water:
Ø Water is a polar solvent and it can dissolve all polar molecules in it.
Ø Important polar molecules of biological interest are ions, salts, sugars, some amino acids and nucleic acids.
Ø When ions or polar molecules come in contact with water, they are surrounded by the water molecules.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Ø This interaction causes the separation of ions from each other.
Ø This is what happens when a substance such as a sugar dissolves in water.
Ø This interaction also increases the mobility of molecules or ions than it was in the solid state.
Ø Increase in mobility makes the ions or molecules more chemically reactive.
Ø Since water can dissolve the majority of the biological molecules, it is called as a universal solvent or the ‘solvent of life’.
Ø Non polar molecules cannot dissolve in water and they tend to move away from water (hydrophobic or water hating).
Ø Lipids, fats, fatty acids and some amino acids are the important non polar molecules of biological interest.
Ø Hydrophilic and hydrophobic interactions of cellular macro and micro molecules are very important in cell physiology and metabolism.
Biological significance of solvent properties of water:
Ø Water acts as a medium for the reactants to undergo chemical reaction in the presence of biological catalysts called enzymes.
Ø Formation of cell membrane and the three dimensional structures of protein and enzymes are due to the hydrophilic and hydrophobic interactions of different class of molecules with water.
Ø Solvent properties of water also make it as a transport medium in the cells.
Ø Example: Blood and lymphatic transport, Alimentary canal transport, Excretory system in animals, Absorption and transport of minerals in plants and Transport of food materials through the phloem of plants.
(2). The high heat capacity of water:
Ø Heat capacity: the amount of heat energy required to raise the temperature of 1 kg of water by 1oC.
Ø Water has high heat capacity.
Ø This means that high amount of energy input is required to raise the temperature of water.
Ø High heat capacity of water is due to the presence of large number of hydrogen bonds in the liquid water.
Ø The energy input is used in the breaking of hydrogen bonds.
Biological significance of high heat capacity of water:
Ø Due to the high heat capacity of water, the rapid temperature fluctuations are minimized in water.
Ø Thus all biological process can operate at a small temperature range.
Ø High heat capacity of water also provides a relatively constant environment in the cell for all chemical reactions occurs inside the cell.
(3). High heat of vaporization:
Ø Heat of vaporization: heat energy required to vaporize a liquid.
Ø It is the energy required to break the attractive forces between the molecules.
Ø It is also the energy required to convert liquid phase of a molecule to its gaseous phase.
Ø In water, the heat of vaporization is the energy required to break the hydrogen bonds in them.
Learn more: How Hydrogen Bonds are Formed in Water?
Ø Water has a high heat of vaporization.
Ø This means, relatively high amount of heat energy is required to vaporize the water.
Ø Due to the high heat of vaporization, the water has high boiling point.
Biological significance of high heat of vaporization of water:
Ø Energy entering to the water is utilized for its vaporization.
Ø This results in the cooling of the surroundings.
Ø This process is utilized in sweating process to reduce the body temperature in animals
Ø Due to the high heat of vaporization of water, the organism can attain a high heat loss with a relatively less amount of water loss.
Ø Transpiration process in plants also cools the leaves and makes them stay in the open sunlight.
(4). Water has high heat of fusion:
Ø Heat of fusion: heat energy required to melt a solid to liquid.
Ø Heat of fusion is also called as melting point.
Ø Melting point of water is 0oC.
Ø 0oC may not feel as a high melting point, but when you see the melting point of other common solvents (in the table below); the zero seems to be a rather higher value.
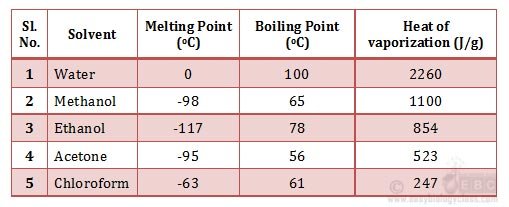
Ø Due to the high heat of fusion, the ice crystals needs relatively high amount of heat energy to thaw.
Ø In other words, the liquid water must lose a relatively high amount of heat energy to freeze.
Biological significance of the high heat of fusion of water
Ø Cell in relatively lower temperature (polar and alpine areas) are less likely to freeze due to the high heat of fusion of water.
Ø Freezing cause crystal formation in the cells.
Ø Ice crystal can break the cell membrane and cell organelles and can kill the cells.
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
(5). Density and freezing properties of water:
Ø The density of liquid water is more than its solid state (ice).
Ø The density of water decreases below 4oC.
Ø Water is the only substance whose solid form is less dense than its liquid state.
Ø Due to the less density of ice than water, they tend to float over the water.
Biological significance of low density of ice over liquid water:
Ø In the water bodies of low temperature areas (polar region) the top region freezes first and the bottom last.
Ø If freezing starts from the bottom to top, the life forms will not survive in the polar region.
Ø The floating ice plates act as an insulator over the water bodies.
Ø This increase the chance of the survival of organism under the water in Polar Regions.
Ø Also, water below 4oC tends to rise to the surface and allow the nutrient cycling in water bodies.
(6). High surface tension and cohesion
Ø Cohesion: force whereby individual molecules of water sticks together.
Ø Water has high cohesive forces.
Ø The cohesion of water is due to the presence of a large number of hydrogen bonds in water.
Ø Surface tension: a force on the surface of a liquid molecule due to the cohesive forces between the molecules.
Ø High surface tension causes a liquid to occupy the least possible surface area.
Ø The least possible surface areas in a liquid are obtained when it is in a spherical shape.
Ø This is the reason for the spherical shape of water drops.
Biological significance of high cohesive force of water:
Ø Cohesive and adhesive forces of water are the main forces of transpiration pull which facilitate the transport of water in plants through the xylem.
(7). Water as a reactant in the cells:
Ø Water is a reactant in the biological system.
Ø Water participates in many chemical reactions.
Ø Water has a slight tendency of ionization.
Ø Water can ionize to release H+ and OH– ions.
Learn more: Ionization of Water an pH Scale
Ø Water is the source of H+ ions in photosynthesis.
Ø Water is a substrate in the hydrolysis reactions taking place in the cells.
Ø Water molecules are eliminated during the condensation reaction, a common type of polymerization reaction taking place in the cells such as during protein synthesis, synthesis of polysaccharides and synthesis of fats from glycerol and fatty acids.
You may also like…
@. Ionization of Water and pH Scale
@. How Hydrogen Bonds are Formed in Water
@. Biological Significance of Water

The note is clear
Thank you, Keep visiting easybiologyclass