The development of mRNA vaccines against infectious diseases is a significant milestone in the history of medicine. These vaccines have global attention for their extraordinary speed in addressing the COVID-19 pandemic. This article explores the groundbreaking science behind mRNA vaccines, their development, mechanisms of action, advantages, challenges, and the potential for transforming the future of vaccination.
What is mRNA Vaccine?
Ø An mRNA vaccine is a type of vaccine that uses a copy of messenger RNA (mRNA) to produce an immune response.
Ø Messenger RNA (mRNA) vaccines represent a novel approach to vaccination.
Ø Foundational work on use of mRNA as vaccine was laid by Katalin Karikó and Drew Weissman.
Ø They pioneered the development of modified mRNA molecules that could be used safely in humans.
Ø Their groundbreaking research paved the way for the development of mRNA vaccines against various infectious diseases.
Ø The 2023 Nobel Prize in Physiology and Medicine was awarded to Katalin Karikó and Drew Weissman for their contributions in the development of mRNA vaccines against COVID-19.
Eples of mRNA vaccinesxam
(1). Pfizer–BioNTech COVID-19 vaccine sold under the trade name Comirnaty
(2). Moderna COVID‑19 vaccine sold under the trade name Spikevax
(3). CureVac COVID-19 vaccine
(4). Walvax COVID-19 vaccine
Mechanism of Action of mRNA Vaccines
Ø The Pfizer-BioNTech and Moderna COVID-19 vaccines are examples of mRNA vaccines that work by injecting a small amount of artificially produced mRNA into the body.
Ø This mRNA codes for a protein called a spike that is located on the surface of the SARS-CoV-2 virus.
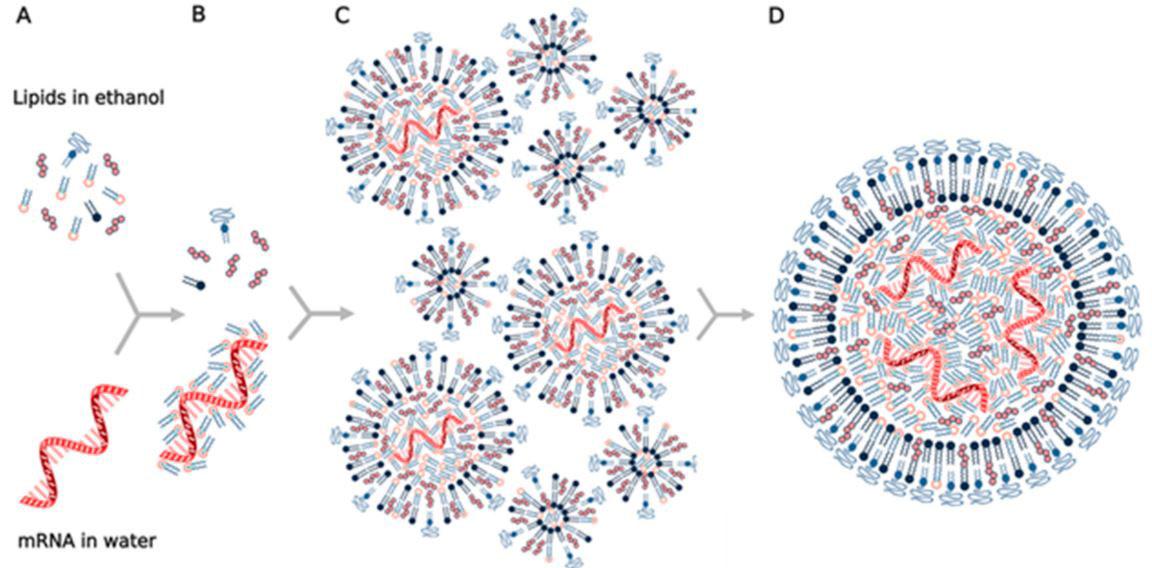
Ø The mRNA is delivered by a co-formulation of the mRNA encapsulated in lipid nanoparticles.
Ø The lipid nanoparticles protect the RNA strands and help their absorption into the cells.
Ø These mRNA fragments are taken up by dendritic cells through phagocytosis.
Ø Once the mRNA enters cells, ribosomes use it as a template to produce the spike protein.
Ø Once the viral antigens are produced by the host cell, the normal adaptive immune system processes are followed.
Ø Antigens are first broken down by proteasomes.
Ø The Class I and class II MHC molecules then attach to the antigen and transport it to the cellular membrane, “activating” the dendritic cell.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Ø Once activated, dendritic cells migrate to lymph nodes, where they present the antigen to T cells and B cells.
Ø This triggers the production of antibodies specifically targeted to the antigen, ultimately resulting in immunity.
Ø Most importantly, the immune response is not directed against the whole virus, making the vaccine safe.
Advantages of mRNA Vaccines
The advantages of mRNA vaccines over traditional vaccines are ease of design, speed and lower cost of production, the induction of both cellular and humoral immunity, and lack of interaction with the genomic DNA.
Speed
Ø mRNA vaccine development is faster than traditional methods.
Ø It does not require growing the virus in the lab.
Ø This speed was especially crucial during the COVID-19 pandemic, enabling the rapid creation of effective vaccines.
Safety
Ø mRNA vaccines do not contain live or weakened viruses.
Ø This eliminated the risk of causing the disease they are designed to protect against.
Ø Adverse reactions are generally mild and transient.
Flexibility
Ø The same mRNA platform can be adapted to target different pathogens, making it a versatile tool for addressing various infectious diseases.
Scalability
Ø mRNA vaccines are produced using cell-free systems, allowing for rapid scaling of production to meet global demand.
Challenges and Concerns
While mRNA vaccines offer numerous advantages, they are not without challenges and concerns. Some of them are summarized below:
Storage and Distribution
Ø Most mRNA vaccines like Pfizer-BioNTech’s vaccine requires ultra-low temperatures for storage, posing logistical challenges in some regions.
Long-Term Safety
Ø Reactogenicity (tendency of a vaccine to produce adverse reactions) of mRNA vaccine is like that of conventional non-RNA vaccines.
Ø People susceptible to an autoimmune response may have an adverse reaction to messenger RNA vaccines
Ø The long-term safety of mRNA vaccines is still being studied, as they are relatively new.
Ø Ongoing monitoring is essential to assess any rare or unexpected adverse events.
Vaccine Uncertainty
Ø Effective communication and education are crucial, as certain people have become skeptical due to the swift development of mRNA vaccines.
Future of mRNA Vaccines
Ø mRNA vaccines have proven successful against COVID-19, ushering in a new era in immunization research.
Ø Scientists are now investigating the vaccines’ ability to fight HIV, influenza, and other infectious diseases.
Ø Moreover, mRNA technology can be applied to develop customized cancer vaccines that adjust medication dosages based on each patient’s particular genetic composition.
Conclusion
mRNA vaccines, which provide a flexible, safe, and effective way to fight infectious diseases, are a breakthrough in the science of immunization. The COVID-19 pandemic has shown how remarkably capable they are of reacting quickly to new threats. Even if there are still obstacles, more study and development could lead to even more opportunities for mRNA vaccines in the future.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |