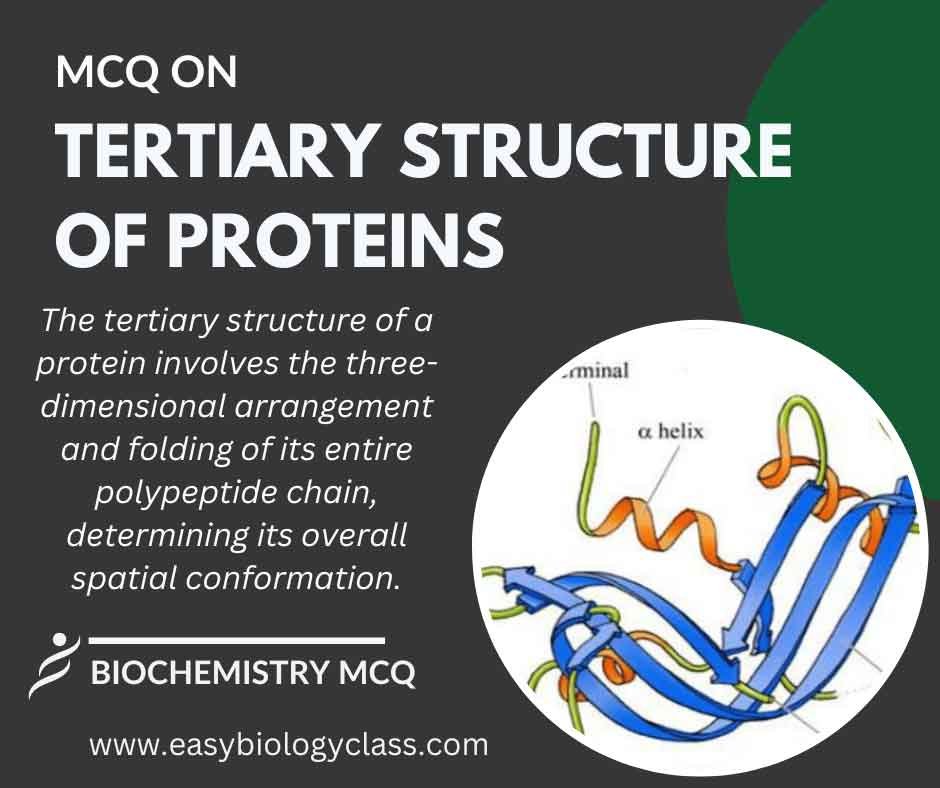
Tertiary structure refers to the overall three-dimensional arrangement of a single polypeptide chain in a protein. It results from the folding and interactions between distant amino acid residues, forming a complex, functional structure crucial for the protein’s biological activity. This is an MCQ on Tertiary Structure of Proteins.
| Biochemistry Notes | Biochemistry PPT | Biochemistry MCQs |
<<< Back to Biochemistry MCQ Page
You may also like...
NOTES QUESTION BANK COMPETITIVE EXAMS.
PPTs UNIVERSITY EXAMS DIFFERENCE BETWEEN..
MCQs PLUS ONE BIOLOGY NEWS & JOBS
MOCK TESTS PLUS TWO BIOLOGY PRACTICAL