Learning objectives: Origin of Earth, Biochemical Origin of Life, Formation of Micro and Macro-molecules, Coacervates and Coacervation, Prions and Protenoids, Formation of Pro-cell, Formation of Cell, Oparin and Haldane theory, Miller and Urey’s Experiment
Biochemical Origin of Life
Ø Biologists believe that present-day life was originated in the remote past from chemical substances by ‘Biochemical Evolution’ or ‘Chemical Evolution’.
Ø Biochemical Evolution: The formation of complex organic molecules from simpler inorganic molecules through chemical reactions in the oceans during the early history of the Earth.
Ø Biochemical evolution was the first step in the development of life on earth.
Ø The period of chemical evolution lasted for about a billion years.
Ø This theory is also called as Molecular Evolution or Chemical Evolution or Modern Theory of Origin of Life
Ø It is a new version of ‘Abiogenesis’.
Ø The theory of Biochemical Origin of Life was:
$. Proposed by Oparin & Haldane (1924).
$. Evidences provided by Urey & Miller (1953).
Ø The theory explain that:
$. Life originated from simple inorganic substances.
$. Inorganic substances are transformed to organic substances.
You may also like NOTES in...
BOTANY BIOCHEMISTRY MOL. BIOLOGY
ZOOLOGY MICROBIOLOGY BIOSTATISTICS
ECOLOGY IMMUNOLOGY BIOTECHNOLOGY
GENETICS EMBRYOLOGY PHYSIOLOGY
EVOLUTION BIOPHYSICS BIOINFORMATICS
$. Organic substances later transformed into colloidal substances.
$. Colloidal substances acquired life (capacity of propagation).
$. The colloidal substances with ‘life’ behaved like present-day prokaryotic cells.
Origin of life on Earth includes following processes in a sequential way:
(1). Origin of Earth
(2). Formation of water, Ammonia and Methane
(3). Formation of Micro-molecules
(4). Formation of Macro-molecules
(5). Formation of Nucleic Acids
(6). Formation of Nucleoproteins
(7). Coacervation
(8). Pre-cell or Pro-cell formation
(9). Cell Formation
(1). Origin of Earth
Ø Origin of planet earth occurred before the origin of life.
Ø It is believed that earth formed from the sun about 4.54 billion years ago.
Ø A piece of sun broken from the sun.
Ø The piece then gradually moves away from the sun.
Ø The broken piece was a firewall.
Ø It contains molten mass of gasses and vapours of various elements.
Ø The temperature was very high (about 5000 to 6000oC).
Ø As the earth moved away from the sun, it gets cooled.
Ø Heavier elements such as Ni and Fe occupied at the core of the earth.
Ø Lighter elements such as He, H, O, N and C occupied the atmosphere of the earth.
Ø The concentration of Molecular oxygen (O2) was very less or absent.
Ø Life originated mainly from elements occupied in the atmosphere.
Ø These elements underwent a series of progressive changes for millions of years.
Ø These changes resulted in the origin of life on earth.
Ø Such a type of origin of life from chemical substances is called chemical evolution.
Ø The energy required for the chemical reactions were obtained from sunlight, lightning and volcanic activities.
(2). Formation of Water, Ammonia and Methane
Ø Primitive earth atmosphere contain a large amount of H, N, C and few O.
Ø Hydrogen reacted with other atoms to form a variety of molecules.
$. Hydrogen combined with Nitrogen to form Ammonia (NH3).
$. Hydrogen combined with Carbon to form Methane (CH4).
$. Hydrogen combined with Oxygen to form Water (H2O).
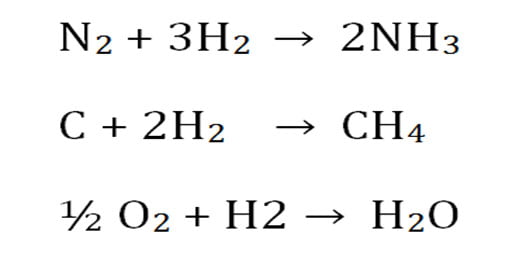
Ø As the years passed, the earth’s atmosphere gradually cooled.
Ø Steam of water in the atmosphere condensed to liquid water.
Ø This resulted in the formation of rain (precipitation).
Ø Since the earth was hot when the rainwater reaches the earth surface, it gets re-evaporated immediately.
Ø This again produces rain in the next cycle.
Ø This process of re-evaporation and raining continued for many years.
Ø This gradually resulted in the cooling of the earth surface.
Ø Rain water gets accumulated on the earth surface.
Ø This resulted in the formation of rivers, streams, lakes and oceans.
Ø Compounds like methane, ammonia get dissolved in the rainwater.
Ø They reached the earth surface and accumulated in the ocean.
Ø Minerals rocks of the earth surface were also dissolved in water.
Ø This resulted in the accumulation of minerals in the seawater.
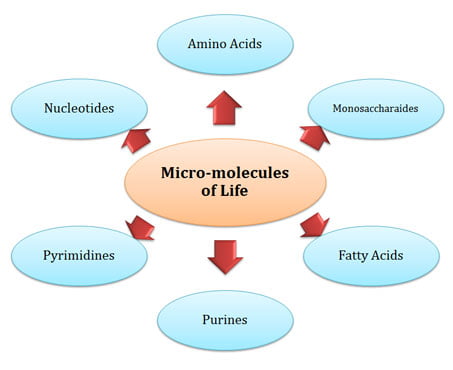
(3). Formation of Micro-molecules
Ø Molecules which are of less size are called micro-molecules.
Ø Major micro-molecules required for the formation of life on earth are:
$. Amino Acids
$. Fatty Acids
$. Purines
$. Pyrimidines
$. Nucleotides
Ø They are formed by the combination of already formed compounds (Ammonia, Methane etc.) and elements (accumulated in the ocean).
Ø Their combinations and reactions required high energy input.
Ø The energy was obtained from sunlight, lightning and volcanic activities.
(4). Formation of Macro-molecules (Condensation or Polymerization)
Ø Macro-molecules were formed by the combination of micro-molecules.
Ø Important Macro-molecules of life are:
$. Proteins: Formed by the polymerization of amino acids.
$. Lipids: Formed by the polymerization of alcohols and fatty acids.
$. Polysaccharides: Formed by the polymerization of monosaccharaides.
$. Nucleic acids: Formed by the polymerization of purines, pyrimidines and phosphoric acid and sugars
Ø The combination process of micro-molecules to form macro-molecules is called condensation or polymerization.
Ø Proteins may be the first macro-molecule formed during the biochemical evolution.
Ø First formed proteins were called proto-proteins.
Proto-proteins
Ø Proto-proteins were the first formed proteins during the chemical origin of life on earth.
Ø All the present-day proteins were evolved from the ‘proto-proteins’
Ø Proto-proteins were small proteins of 15-20 amino acid residues in size.
Ø They have some features of the secondary structures.
Ø They may have some primitive functions.
Ø During the course of time, increasingly stable and more functional proteins arose by adding structural elements to the proto-proteins.
Chemical origin of Life PPT (Download)
Protenoids
Ø They are also called as Thermal Proteins.
Ø Protenoid is a polypeptide or mixture of polypeptides obtained by heating a mixture of amino acids.
Ø They are protein-like, often cross-linked molecules.
Ø They have formed abiotically from amino acids.
Ø According to Sidney W. Fox protenoids may have been the precursors to the first living cells (proto-cells).
(5). Formation of nucleic acids
Ø Nucleic acids were formed by the condensation of phosphoric acid, sugars, purine and pyrimidine.
Ø First nucleosides were formed.
Ø Then nucleotides were formed.
Ø Nucleotides polymerized to form nucleic acids (RNA and DNA).
Ø RNA was formed first, DNA was formed later from RNA.
Ø RNA was the first genetic material.
Ø Later RNA as the genetic material was replaced by DNA.
Ø DNA is more stable than RNA.
RNA World Hypothesis
Ø Proposed by Alexander Rich in 1962
Ø Description: RNA world is a hypothetical stage in the evolutionary history of life on Earth, in which self-replicating RNA molecules proliferated before the evolution of DNA and proteins. Like DNA, RNA can store and replicate genetic information; like protein enzymes, RNA enzymes (ribozymes) can catalyze (start or accelerate) chemical reactions that are critical for life. One of the most critical components of cells, the ribosome, is composed primarily of RNA.
(6). Formation of nucleoproteins
Ø Proteins and nucleic acids combined to form nucleoproteins.
Ø Nucleoproteins started to perform specific functions.
Ø Enzymes like activities were acquired by these molecules.
Ø Nucleoproteins were more stable than nucleic acid alone.
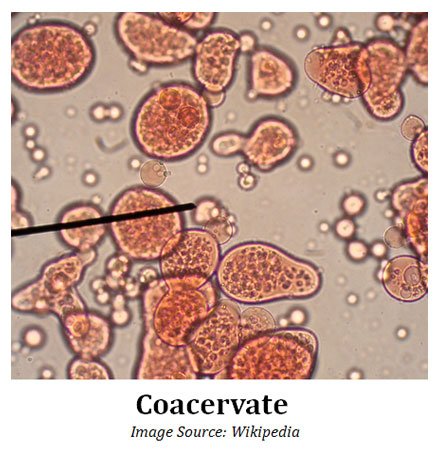
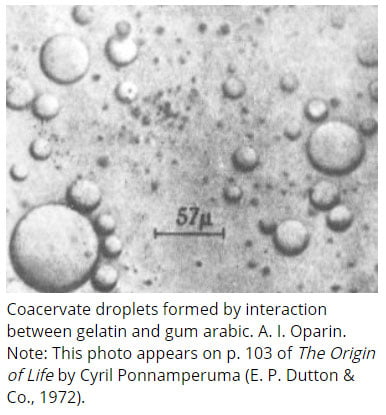
(7). Coacervates and Coacervation
Ø Word meaning: ‘To Assemble Together’
Ø Coacervates: They are organic-rich droplets formed via liquid-liquid phase separation, mainly resulting from association of oppositely charged molecules (polysaccharides, proteins, macro-ions) or hydrophobic proteins.
Ø Coacervation: A phenomenon that produces coacervate colloidal droplets.
Ø When coacervation occurs, TWO liquid phases will co-exist.
Ø The TWO phases are:
$. A dense, polymer-rich phase (coacervate phase).
$. A very dilute, polymer-deficient phase (dilute phase).
Ø Process of coacervation was proposed by Oparin and Haldane
Ø Macromolecules formed by the polymerization of micro-molecules underwent precipitation in the sea.
Ø Precipitation resulted in the aggregation of macro-molecules.
Ø The aggregation resulted in the formation of organized structures.
Ø These organized structures can be called as Coacervates.
Ø Coacervates are distinct colloidal droplets appeared in the sea.
Ø Smaller coacervates fused together to form larger coacervates.
Ø Coacervates were NOT mixed with the surrounding water.
Ø Coacervates contained protein, nucleic acids and other organic and inorganic compounds.
Ø The ratio of these compounds in the coacervates highly varies.
Ø Surface of coacervates had the ability to selectively absorb other substances from the medium (seawater).
Ø According to Oparin, coacervates behaved like living molecule.
Ø He also suggested that coacervates may give rise to cell-like structures.
Ø Coacervates were also called as microspheres.
Ø Coacervates or Microspheres show features of a living cell:
$. They were microscopic in size
$. Highly dynamic
$. Spherical in shape
$. Had a uniform diameter
$. They were stable in water
$. Had double-layered boundaries around them
$. Had mobility
$. They showed growth
$. They underwent fission, budding and fragmentation
(8). Proto-cell Formation
Ø First living cell is called Proto-cells or Pro-cell or Proto-biont.
Ø Proto-cells originated spontaneously in the seawater.
Ø Proto-cells were spherical in shape.
Ø They had double-layered membrane around it.
Ø Had the capacity to reproduce by fission, budding or fragmentation.
Ø Had the capacity to take materials from surrounding.
Ø Obtain energy from the fermentation of organic substances. Thus, proto-cells were anaerobes.
(9). Cell formation
Ø The proto-cells gave rise to cells.
Ø Proto-cells acquired genetic material (RNA or DNA or nucleoprotein).
Ø Most probably the genetic material may be RNA.
Ø They looked like modern bacteria or virus.
Ø RNA or DNA acquired the self-replicating capacity.
Ø Genetic material started to assist protein synthesis.
Ø Genetic material acquired replication capacity.
Ø Earlier cells obtain energy from fermentation
Ø Fermentation released a large amount of CO2 to the atmosphere
Ø CO2 accumulated in the atmosphere.
Ø Continuous fermentation resulted in the exhausting of resources.
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
Ø This condition might be responsible for the development of chlorophyll.
Ø Chlorophylls started to capture CO2 and fix light energy.
Ø Photosynthesis produced O2 as a byproduct.
Ø O2 produced by photosynthesis is released into the atmosphere.
Ø With this, the heterotrophic cells were transformed into autotrophic cells.
Ø When life was originated, the atmosphere was anaerobic.
Ø Molecular oxygen was very less or absent.
Ø The life process gradually changed the anaerobic condition to aerobic.
Place of origin of life on earth
Ø ‘Sea is the mother of life’.
Ø Majority of scientists believed that life originated in the water in the sea.
Ø In the sea, life originated at deep hydrothermal vents.
Ø This view is supported by many facts:
$. Most of the simple and primitive animals and plants are aquatic
$. Protoplasm and body fluids contain salt similar to sea
$. Earliest fossils were obtained from the sea bed
Do you have any Queries?
Please leave me in the Comments Section below.
I will be Happy to Read your Comments and Reply.