Colloids vs Crystalloids
Difference between Colloids and Crystalloids: This article is about the Difference between Crystalloids and Colloids with a comparison table. First lets see what are Colloids and Crystalloids.
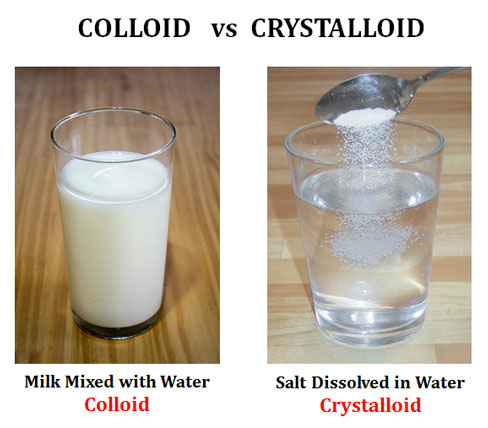
Colloids: Colloids are homogeneous non-crystalline substances containing large molecules or ultramicroscopic particles of one substance dispersed in a second substance. Colloids include gels, sols, and emulsions. Unlike the suspension, the particles in the colloid do not settle and they cannot be separated out by ordinary filtering or centrifugation.
Crystalloids: Crystalloids are aqueous solutions of salts or minerals that can be crystallized.
Thus the main difference between colloids and crystalloids are their particle size. Both colloids and crystalloids are used as volume expanders and hence have immense applications in the medical field.
You may also like NOTES in...
BOTANY BIOCHEMISTRY MOL. BIOLOGY
ZOOLOGY MICROBIOLOGY BIOSTATISTICS
ECOLOGY IMMUNOLOGY BIOTECHNOLOGY
GENETICS EMBRYOLOGY PHYSIOLOGY
EVOLUTION BIOPHYSICS BIOINFORMATICS
Difference between Colloids and Crystalloids
Sl. No. Colloids Crystalloids
1 Colloids are those substances which are not easily crystallized from their aqueous solutions. Crystalloids are those substances which are easily crystallized from their aqueous solution.
2 Example: starch, gelatin, gum Example: salt, sugar, urea
3 Colloids contain much larger particles than crystalloids (1 – 200 nm). Crystalloids contain much smaller particles than colloids (<1 nm).
4 Vascular permeability of colloids is comparatively low. Vascular permeability of crystalloids is high.
5 Colloids do not pass through the plasma membrane of the cell. Crystalloids quickly pass through the plasma membrane of the cell.
6 Colloids are heterogeneous systems. Crystalloids are homogenous systems
7 Colloids are classified as lyophilic and lyophobic colloids. Crystalloids are classified as isotonic, hypertonic and hypotonic.
8 Colloids should be stored in refrigerators. Crystalloids can be stored at room temperature.
9 Preparation of colloids is tedious. Preparation of crystalloids is very easy.
10 Colloids cannot act as the source of electrolytes. Crystalloids can act as a source of electrolytes in the body.
11 Colloids have short life span. Crystalloids have long life time.
<< Back to Plant Physiology Notes Page
