Covalent Bonds vs Ionic Bonds
Difference between Ionic Bond and Covalent Bond: The chemical bonds are stable attractions between atoms, ions or molecules. The formation of chemical bond allows the formation of molecules or compounds. Chemical bonds are classified into different categories based on their formation and strength. Important types of chemical bonds recognized are Covalent bonds, Ionic bonds, Metallic bonds, Dipole-dipole interactions, London dispersion forces and Hydrogen bonds.
The present post discuss about the Similarities and Differences between the Covalent bond and Ionic bond with a Comparison Table.
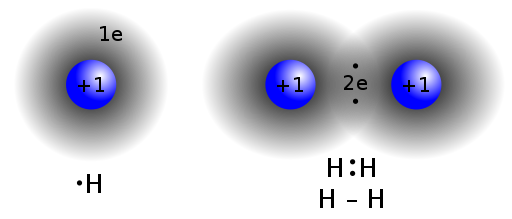
Covalent bond: A chemical bond formed by the sharing of electron pairs.
Ionic bond: A chemical bond formed by the electrostatic attraction between oppositely charged ions.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Similarities between Covalent Bond and Ionic Bond
Ø Both covalent and ionic bonds are strong bonds.
Ø Both are primary bonds.
Ø Both bonds result in the formation of complex structures.
Ø The formation of both covalent and ionic bonds results in the formation of more stable compounds than the original.
Ø Formation of covalent and ionic bonds is exothermic.
Ø Compounds formed by both covalent and ionic bonds will have net neutral charge.
Ø Compounds with both covalent and ionic bonds are not malleable.
Difference between Ionic Bond and Covalent Bond