Learning Objectives: How Enzymes are Named? What is IUB System, Components of Enzyme Nomenclature, Seven Major Classes of Enzymes with Examples, What is Enzyme Commission Number? How enzymes are named? Ø Enzymes are classified based on the reaction they catalyze. Ø Usually, the enzymes are named by adding the suffix […]
Continue ReadingTag Archives: Biochemistry Lecture Notes
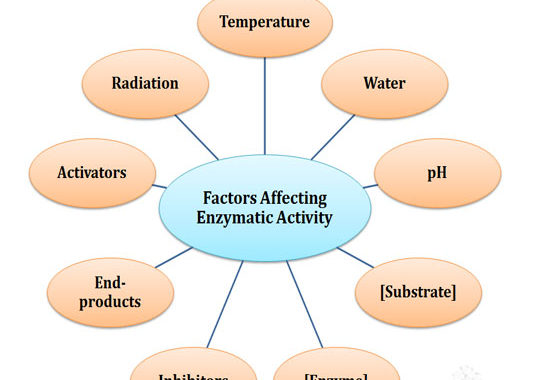
Factors Affecting Enzymatic Activity (Biochemistry Lecture Notes)
In the previous post, we have discussed the Properties of Enzymes. In the present post, we will see what all are the factors that affect the catalytic activity of an enzyme in the living system. Factors Affecting Enzymatic Activity Ø The catalytic activities of enzymes are affected by multiple factors. […]
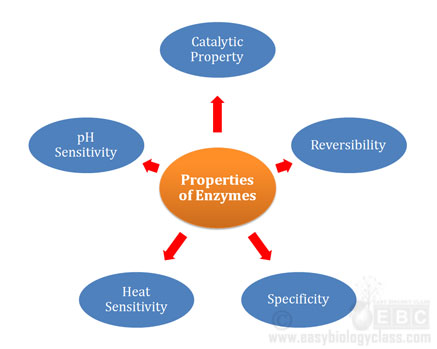
Continue ReadingProperties of Enzymes (Biochemistry Lecture Notes)
Enzymes are biological catalysis. They are specialized proteins (except ribozymes) capable of catalyzing specific reactions in the cells. In the previous post, we have discussed the Structure and Functions of Enzymes. In the present post, we will discuss the Properties of Enzymes. What are the Properties of Enzymes? @. The […]
Continue ReadingIntroduction to Enzymes: Structure and Characteristics-Short Lecture Notes
What are Enzymes? There are thousands of chemical reactions in a living system. The chemical reactions in the cell are catalyzed by the biological catalysts called enzymes. Almost all enzymes are highly specialized proteins. (Exception: Ribozymes –Ribozymes are RNA with catalytic activity). The current post we will discuss the Characteristics […]
Continue ReadingStructure of Proteins (Biochemistry Lecture Notes)
Protein Structure Learning objectives Structure of Proteins: Protein Structure: Primary Structure, Secondary Structure (Alpha Helix, Beta Plates, Beta Turns), Tertiary Structure, Quaternary Structure, Bonds Stabilizing different Protein Structures. Protein Structure Ø Proteins are the polymers of amino acids. Individual amino acids (residues) are joined by peptide bonds to form the […]
Continue Reading