.“There is more to life than increasing its speed…” (Mahatma Gandhi)
Enzymes are biological catalysts. They accelerate the rate of metabolic reactions in the cells by reducing the activation energy of the reactants. Almost all enzymes are specialized proteins with definite structural conformations.
Important characteristic features of enzyme are:
(1). Catalytic Power (ratio of enzyme catalyzed rate of a reaction to the un-catalyzed rate)
(2). Regulation (control of enzymatic reaction)
(3). Specificity (Selectivity of enzyme to their substrate)
What is enzyme specificity?
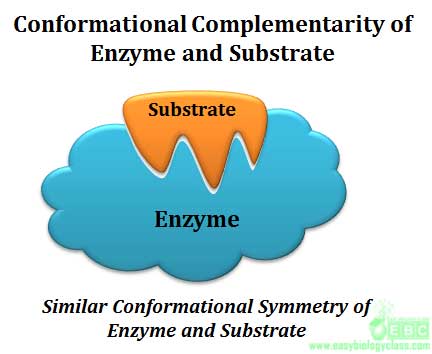
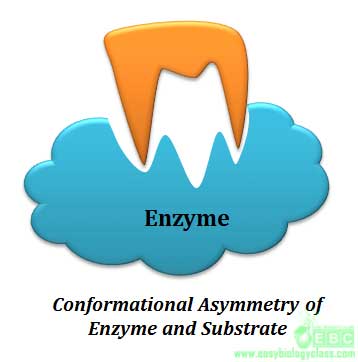
Specificity is the ability of an enzyme to choose exact substrate from a group of similar chemical molecules. The specificity is actually a molecular recognition mechanism and it operates through the structural and conformational complementarity between enzyme and substrate. Enzymes show different degrees of specificity towards their substrate.
 |
.. |  |
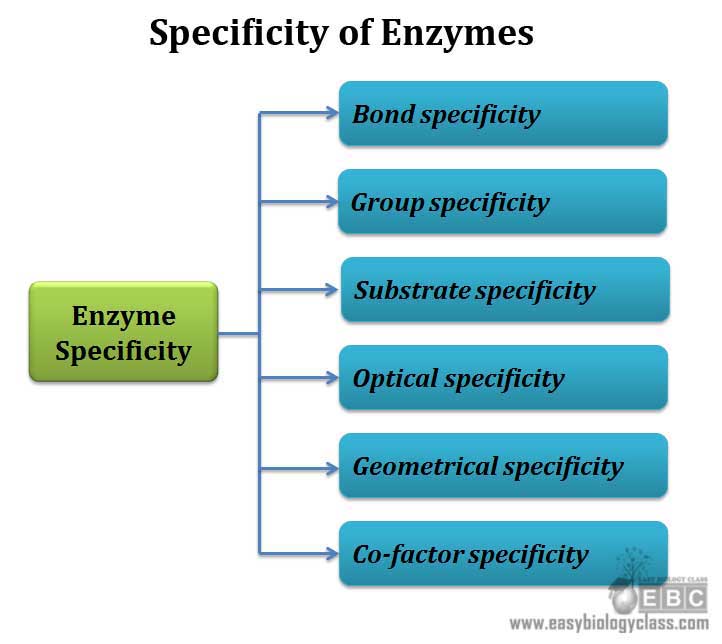
The specificity shown by enzymes are grouped into 6 categories
1. Bond specificity
2. Group specificity
3. Substrate specificity
4. Stereo specificity (Optical specificity)
5. Geometrical specificity
6. Co-factor specificity
 .(1). Bond specificity:
.(1). Bond specificity:
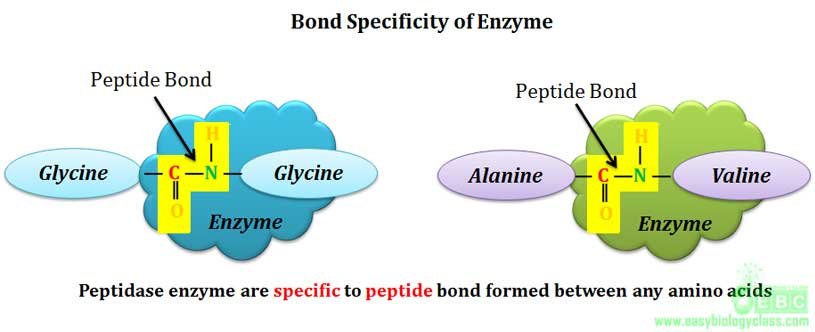
Bond specificity is also called as relative specificity. Enzymes showing bond specificity are specific to substrates having similar bonds and similar structure. This means that they are specific only to certain types of bonds such as peptide bonds, glycosidic bonds, ester bonds etc.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Example: α-amylase enzyme can hydrolyze α-1-4 glycosidic linkage in starch and glycogen, i.e., the enzyme is specific only to α-1-4 glycosidic bond and not to the substrate. Similarly lipase can hydrolyze ester bonds formed between glycerol and any fatty acid. Proteinases are another class of enzymes showing bond specificity, they can hydrolyze peptide bonds formed by any amino acid in the protein.
 .(2). Group specificity:
.(2). Group specificity:
It is also called moderate specificity. Here the enzyme is specific to a bond and groups surrounding the bonds. Group specificity is more than that of bond specificity. Endopeptidases and exopeptidases (two general classes of proteinases) are classical examples for group specificity.
Example:
(1). Pepsin, a digestive enzyme of stomach produced by chief cells, can hydrolyze a peptide bond in which the amino group is contributed by an aromatic amino acid such as phenyl alanine, tyrosine and tryptophan..
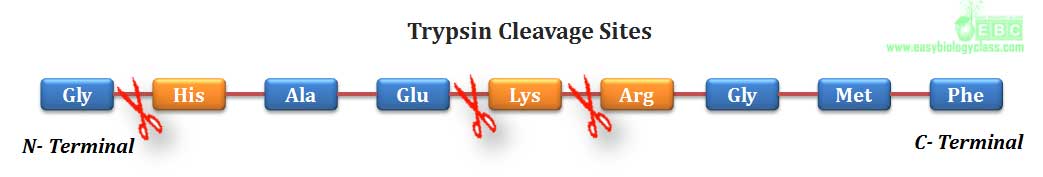
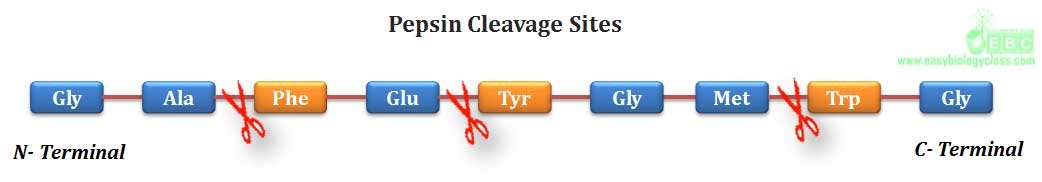 .(2). Trypsin, a serine protease of digestive system, can hydrolyze a peptide bond in which amino group is contributed by any basic amino acid such as lysine, arginine and histidine.
.(2). Trypsin, a serine protease of digestive system, can hydrolyze a peptide bond in which amino group is contributed by any basic amino acid such as lysine, arginine and histidine.
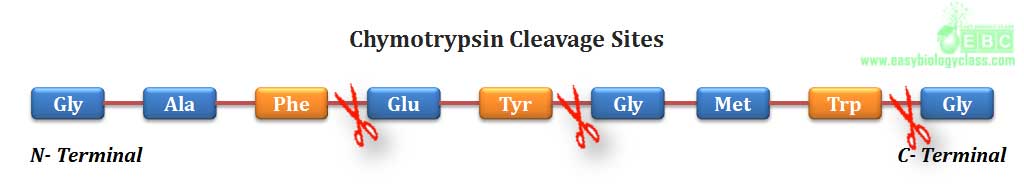
 .(3). Chymotrypsin, a digestive enzyme component of pancreatic juice, can hydrolyze a peptide bond in which the carboxyl group is contributed by any aromatic amino acid (phenylalanine, tyrosine and tryptophan)
.(3). Chymotrypsin, a digestive enzyme component of pancreatic juice, can hydrolyze a peptide bond in which the carboxyl group is contributed by any aromatic amino acid (phenylalanine, tyrosine and tryptophan)
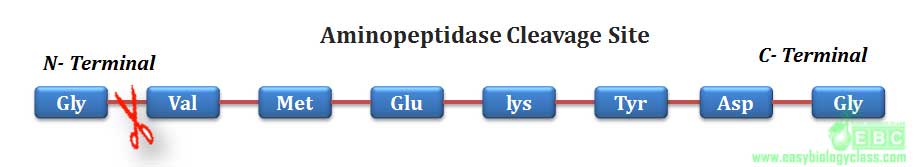
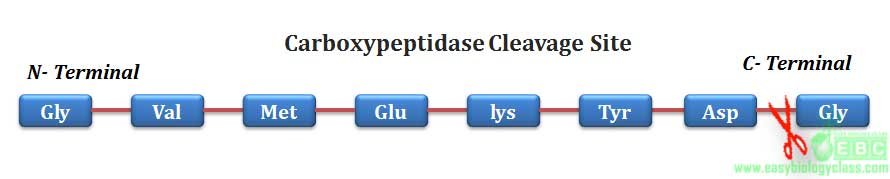
 .Similarly, aminopeptidase and carboxypeptidase can hydrolyze any peptide bond of a protein from N-terminal and C-terminal respectively.
.Similarly, aminopeptidase and carboxypeptidase can hydrolyze any peptide bond of a protein from N-terminal and C-terminal respectively.
 .(3). Substrate specificity
.(3). Substrate specificity
Substrate specificity is also called as absolute specificity, since here the specificity is very high. Enzymes showing substrate specificity are specific only to one substrate and one reaction.
Example: Enzyme lactase can only hydrolyze the β-1-4 glycosidic bond of lactose to yield galactose and glucose. Similarly, Maltase can only act on the α-1-4 glycosidic linkage of two glucose molecules in maltose..
(4). Optical specificity
Optical specificity of enzyme is also called as stereo-specificity. Here the enzyme is specific not only to substrate but also to its optical configuration. Optical specificity of enzyme is considered as the highest specificity shown by any class of enzyme in the living world.
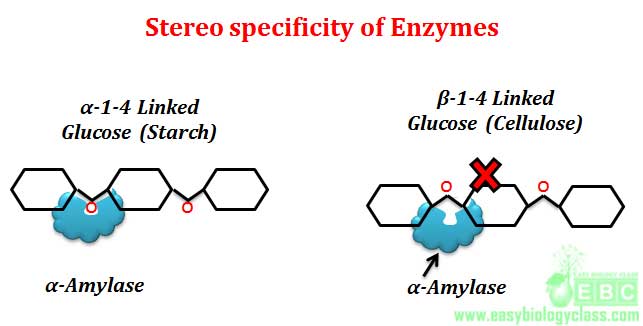
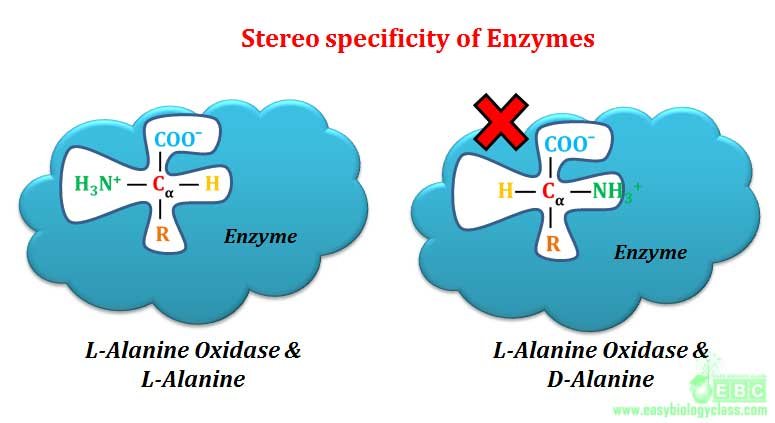 Example: L-amino acid oxidase acts only on L-amino acids, similarly D-amino acid oxidase acts only on D-amino acids. Another good example for optical or stereo specificity of enzyme is the action of amylases. α-glycosidic bonds of starch and glycogen are hydrolyzed only by α-glycosidase (α-amylase). Similarly β-glycosidic bonds of cellulose are hydrolyzed only by β-glycosidase (β-amylase). Starch, glycogen and cellulose are the polymer of glucose molecules joined by 1-4 glycosidic linkages. In starch and glycogen, the connecting bond is α-1-4, where as in cellulose it is β-1-4. Saliva of mammals contain α-amylase enzyme for hydrolyzing starch to release glucose as a source of metabolic energy. Mammals cannot use cellulose as a glucose source because cellulose cannot be hydrolyzed by stereo specific α-amylase which acts only on α-1-4 glycosidic linkage of starch and glycogen.
Example: L-amino acid oxidase acts only on L-amino acids, similarly D-amino acid oxidase acts only on D-amino acids. Another good example for optical or stereo specificity of enzyme is the action of amylases. α-glycosidic bonds of starch and glycogen are hydrolyzed only by α-glycosidase (α-amylase). Similarly β-glycosidic bonds of cellulose are hydrolyzed only by β-glycosidase (β-amylase). Starch, glycogen and cellulose are the polymer of glucose molecules joined by 1-4 glycosidic linkages. In starch and glycogen, the connecting bond is α-1-4, where as in cellulose it is β-1-4. Saliva of mammals contain α-amylase enzyme for hydrolyzing starch to release glucose as a source of metabolic energy. Mammals cannot use cellulose as a glucose source because cellulose cannot be hydrolyzed by stereo specific α-amylase which acts only on α-1-4 glycosidic linkage of starch and glycogen.
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
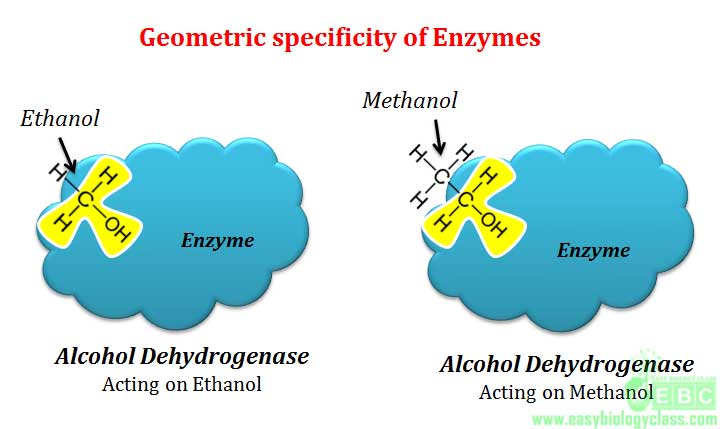
 .(5). Geometrical specificity
.(5). Geometrical specificity
In geometrical specificity, single enzyme can act on different substrates having similar molecular geometry and hence here specificity is very less. Example: Alcohol dehydrogenase can oxidize both ethanol and methanol to yield corresponding aldehydes since both these alcohols have similar molecular geometry.
 .(6). Co-factor specificity
.(6). Co-factor specificity
Co-factors are non-protein part of enzyme required for the functioning of some enzymes. Enzyme which requires co-factors for their activity shows co-factor specificity. Only correct combination of substrate and co-factor allows enzymatic reaction. In the absence of specific co-factor, the enzyme will be inactive even if there are plenty of substrates..
Key Points
Enzyme specificity: Selectivity of enzyme to their substrate
Different types:
1. Bond specificity – specific to bonds
2. Group specificity – specific to bonds and groups surrounding the bonds
3. Substrate specificity – specific to only one substrate and reaction
4. Stereo specificity – specific to substrate and its optical conformation
5. Geometrical specificity – substrates having similar molecular geometry
6. Co-factor specificity – specific to exact substrate, co-factor combination
Different types of specificity and be arranged ascending based on its specificity:
Geometric specificity < Bond specificity < Group specificity < Substrate specificity < Co-factor specificity < Stereo specificity
More Lecture Notes from Easy Biology Class…
