MCQ on Water
(1). Water is liquid at room temperature, the most important reason for this is the:
a. High boiling point of water
b. High melting point of water
c. High heat of vaporization of water
d. Cohesive forces due to hydrogen bonds in water
Learn more: MCQ on pH | MCQ on Buffer Action
(2). Water is a ___
a. Polar solvent
b. Non polar solvent
c. An amphipathic solvent
d. Non polar uncharged solvent
(3). Polar molecules can readily dissolve in water. This is because:
a. Polar molecules can form hydrogen bonds with water
b. Polar molecules can replace water-water interaction with more energetically favourable water-solute interactions
c. Polar charged water can interact with the charge of polar molecules
d. All polar molecules are amphipathic in nature
(4). Most important reason for the unusual properties of water is:
a. The covalent bonding pattern in water molecule
b. The bond angle between the two hydrogen atoms in water
c. Hydrogen bonding between water molecules
d. Water can be immediately ionized at room temperature
(5). The H – O – H bond angle in water molecule is:
a. 104.0o
b. 104.5o
c. 105.0o
d. 105.5o
(6). Which of the following statement is true regarding the electronegativity of atoms in water molecule?
a. Hydrogen is more electronegative than oxygen
b. Hydrogen is less electronegative than oxygen
c. Electronegativity of hydrogen and oxygen is same
d. Oxygen and hydrogen do not have significant electronegativity in water
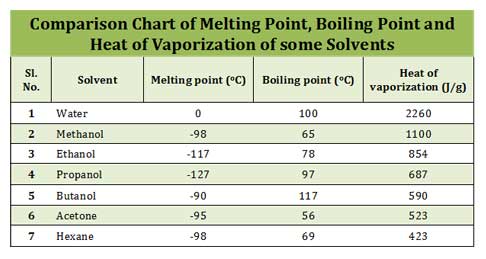
(7). Which of the following represent the current melting point, boiling point and heat of vaporization of water?
a. 0oC ; 100oC ; 2260 J/g
b. 100oC ; 0oC ; 2260 J/g
c. 0oC ; 100oC ; 1260 J/g
d. 100oC ; 0oC ; 1260 J/g
(8). The oxygen atom in the water molecule due to its high electronegativity bears _______
a. 1 δ+ charge
b. 2 δ+ charges
c. 1 δ– charge
d. 2 δ– charges
(9). Hydrogen bond is best represented as the electrostatic attraction between:
a. A hydrogen covalently bounded to an electronegative atom and another hydrogen atom
b. A hydrogen covalently bounded to an electronegative atom and another electronegative atom
c. Two electronegative atoms and a hydrogen atom
d. Two hydrogen atoms
(10). The bond dissociation energy of hydrogen bonds in water molecule is
a. 10 kJ / mol
b. 23 kJ/mol
c. 470 kJ/mol
d. 348 kJ/mol
11. Which of the following statement is correct regarding the hydrogen bonds in water?
a. Hydrogen bond is 10 % covalent and 90 % electrostatic
b. Hydrogen bond is 25% covalent and 75 % electrostatic
c. Hydrogen bond is 50% covalent and 50% electrostatic
d. Hydrogen bond is 100 % electrostatic
12. A single water molecule can form how many hydrogen bonds at a time? (theoretically possible value)
a. 1
b. 2
c. 3
d. 4
13. The life span of a hydrogen bond between two water molecule in liquid water is:
a. 1 – 20 seconds
b. 1 – 20 microseconds
c. 1 – 20 nano-seconds
d. 1 – 20 pico-seconds
14. The bond dissociation energy of O – H bond in water is:
a. 470 kJ/mol
b. 348 kJ/mol
c. 23 kJ/mol
d. 10 kJ/mol
15. What is the bond length of hydrogen bond between two water molecules in liquid water?
a. 0.0177 nm
b. 0.177 nm
c. 1.177 nm
d. 17.70 nm
Please take advantage of our Lecture Notes, PPTs, Previous Year Questions, Mock Tests
You may also like NOTES in...
BOTANY BIOCHEMISTRY MOL. BIOLOGY
ZOOLOGY MICROBIOLOGY BIOSTATISTICS
ECOLOGY IMMUNOLOGY BIOTECHNOLOGY
GENETICS EMBRYOLOGY PHYSIOLOGY
EVOLUTION BIOPHYSICS BIOINFORMATICS
Detailed Answer Key, Explanations and References
1. Ans. (d). Cohesive forces due to hydrogen bonds in water
The physical state of water is determined by the compactness of packing of molecules. The stability of molecular packing depends up on the stability of interactions involved in molecular packing. These interactions will be maximum in solid state (ice in the case of water) and it will be decreased in liquid state (liquid water) and it will be further less in gaseous phase (water vapour). Cohesion is a type of interaction in which the molecules of same types are involved. (Opposite term adhesion: interaction of different types of molecules). The liquid nature of water at room temperature is due to the cohesive forces in the water which is provided by the large number of inter molecular hydrogen bonds between water molecules.
2. Ans. (a). Polar solvent
Water is a polar solvent. Water molecule consists of two hydrogen atoms bonded to an oxygen atom. The two hydrogen atoms in water are not arranged linearly, because the oxygen atom’s four sp3 hybrid orbitals extend roughly toward the corners of a tetrahedron. Hydrogen atoms occupy two corners of the tetrahedron, and the nonbonding electron pairs of the oxygen atom occupy the other two corners. Thus water molecules have an angular geometry. Due to the high difference in the electronegativity difference between oxygen and hydrogen, the oxygen atom with its unshared electrons carries a partial negative charge and the hydrogen atom each carry a partial positive charge. These electrostatic attraction create a diploes and thus the water molecule become polar in nature
3. Ans. (b). Polar molecules can replace water-water interaction with more energetically favourable water-solute interactions
4. Ans. (c). Hydrogen bonding between water molecules
5. Ans. (b). 104.5o
6. Ans. (b). Hydrogen is less electronegative than oxygen
Electronegativity is the affinity of atomic nuclei towards electrons. Each atom has specific electronegativity values, which means that they have different degree of affinity towards electrons. Oxygen, nitrogen etc. are highly electronegative atoms. The electronegativity of hydrogen is very less. When covalent bond is formed between two atoms, one with high electro-negativity and the other with lesser electronegativity, the paired electron cloud in the covalent bond will shift more towards the highly electro-negative atom and this will create a dipole in the molecule.
7. Ans. (a). 0oC ; 100oC ; 2260 J/g
Water is an unusual solvent. Water has got high boiling point (100oC), high melting point (0oC) and high heat of vaporization (2260 J/g) when compared to other solvents.
Heat of vaporization: The heat energy required to convert 1.0 g of a liquid at its boiling point and at atmospheric pressure into its gaseous state at the same temperature. It is a direct measure of the energy required to overcome attractive forces between molecules in the liquid phase.
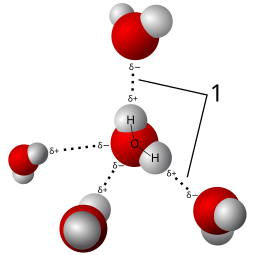
8. Ans. (d). 2 δ– charges
Oxygen is highly electronegative than hydrogen, hence oxygen will pull the shared electron pairs in the covalent bond between O and H more towards it an with this oxygen will get a partial negative charge called δ–. Since there are two hydrogen atoms in water molecule, there will be a total of 2δ– charges in a single water molecule. Similarly, each hydrogen in the water molecule will bear one partial positive charge called δ+.
9. Ans. (b). A hydrogen covalently bounded to an electronegative atom and another electronegative atom
10. Ans. (b). 23 kJ/mol
Hydrogen bond is very weak when compared to covalent bonds. Only 23 kJ/mole of energy is required to break the hydrogen bonds in water. This energy input is very less when compared to the requirement of energy to break the O – H covalent bond in water (470 kJ/mol)
11. Ans. (a). Hydrogen bond is 10 % covalent and 90 % electrostatic
Hydrogen bond in water is 10 percent covalent due to the overlaps in the bonding orbitals and 90 percent electrostatic
12. Ans. (d). 4
A single water molecule can form four hydrogen bonds with four different water molecules in liquid water as shown in the figure below
13. Ans. (d) 1 – 20 pico-seconds
Hydrogen bonds in water are highly dynamic. They life span of each hydrogen bond in water is very short. The lifetime of each hydrogen bond is just 1 to 20 picoseconds ( 1 ps = 10-12S)
14. Ans. (a). 470 kJ/mol
15. Ans. (b). 0.177 nm
Reference: (1). Lehningers Principles of Biochemistry: Chapter: Water
(2). Fundamentals of Biochemistry by Voet and Voet: Chapter: Water
Learn more: MCQ on pH | MCQ on Buffer Action
You may also like...
NOTES QUESTION BANK COMPETITIVE EXAMS.
PPTs UNIVERSITY EXAMS DIFFERENCE BETWEEN..
MCQs PLUS ONE BIOLOGY NEWS & JOBS
MOCK TESTS PLUS TWO BIOLOGY PRACTICAL