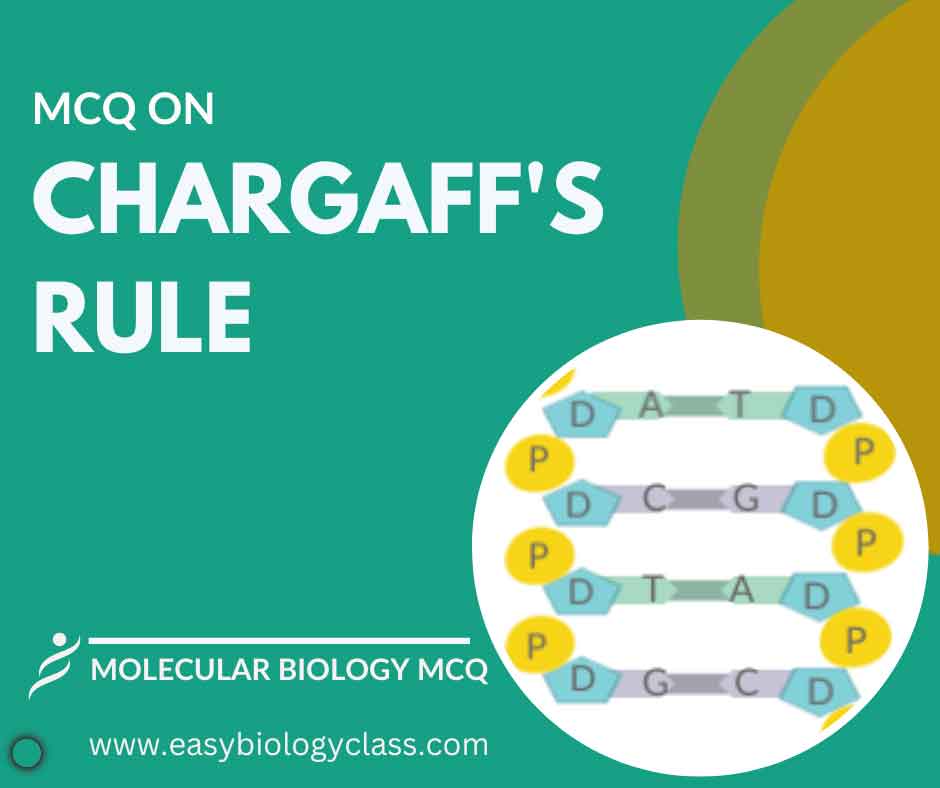
Chargaff’s rule states that in DNA, the amount of adenine (A) equals thymine (T), and the amount of cytosine (C) equals guanine (G). This principle underlies the complementary base pairing in DNA double helix, crucial for genetic stability and replication. This post on Chargaff Rule Questions will help you to […]
Continue ReadingTag Archives: Biochemistry
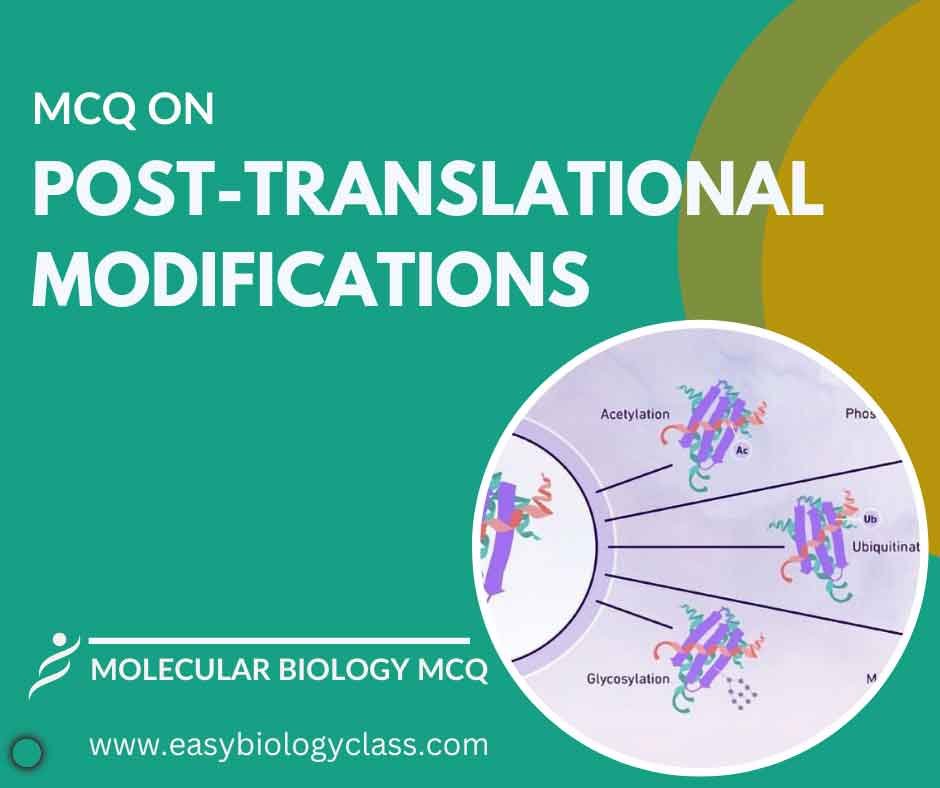
MCQ on Post Translational Modification
Post-translational modifications occur after protein synthesis. They include phosphorylation, glycosylation, and acetylation, altering protein structure and function. These modifications regulate protein activity, localization, and stability, crucial for cellular signaling, metabolism, and gene expression regulation in living organisms. This is an MCQ on Post Translational Modification in Prokaryotes and Eukaryotes. Molecular […]
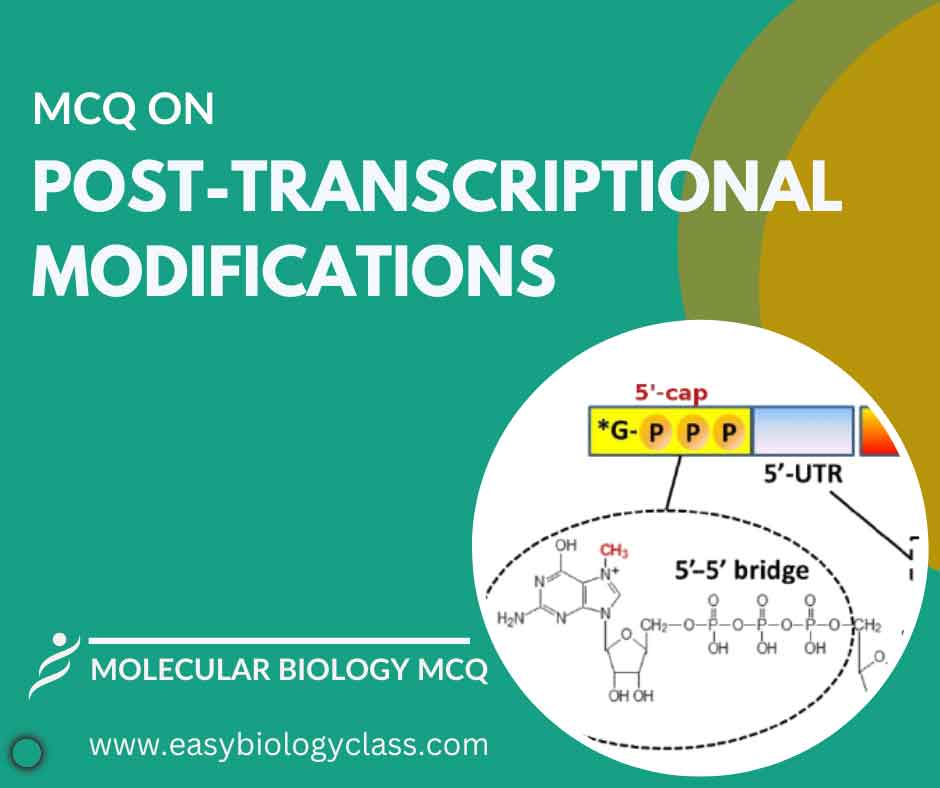
Continue ReadingMCQ on Post Transcriptional Modification
Post-transcriptional modification refers to alterations made to mRNA molecules after transcription but before translation in eukaryotic cells. These modifications include 5′ capping, 3′ polyadenylation, and splicing, which enhance stability, regulate translation, and increase mRNA diversity, essential for proper gene expression. This is an MCQ on Post Transcriptional Modification with answer […]
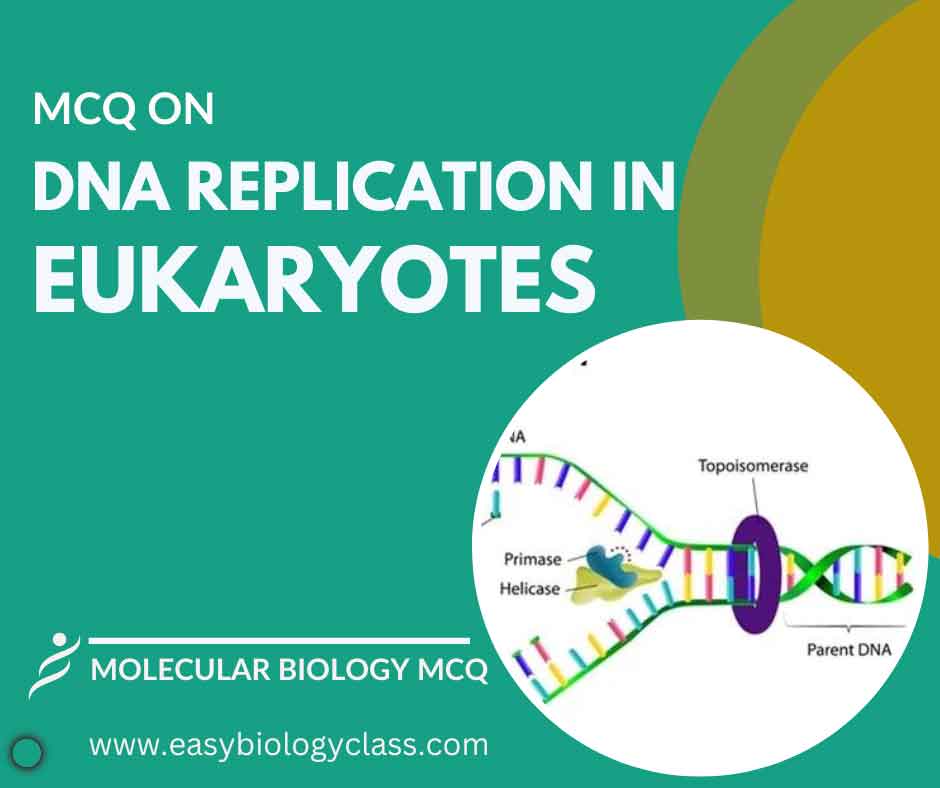
Continue ReadingMCQ on DNA Replication in Eukaryotes
DNA replication in eukaryotes involves initiation at multiple origins, proceeding bidirectionally from replication forks. Enzymes like DNA polymerases synthesize new strands, while helicases unwind the double helix. This process ensures accurate duplication of chromosomes during cell division, vital for genetic stability and inheritance. This is an MCQ on DNA Replication […]
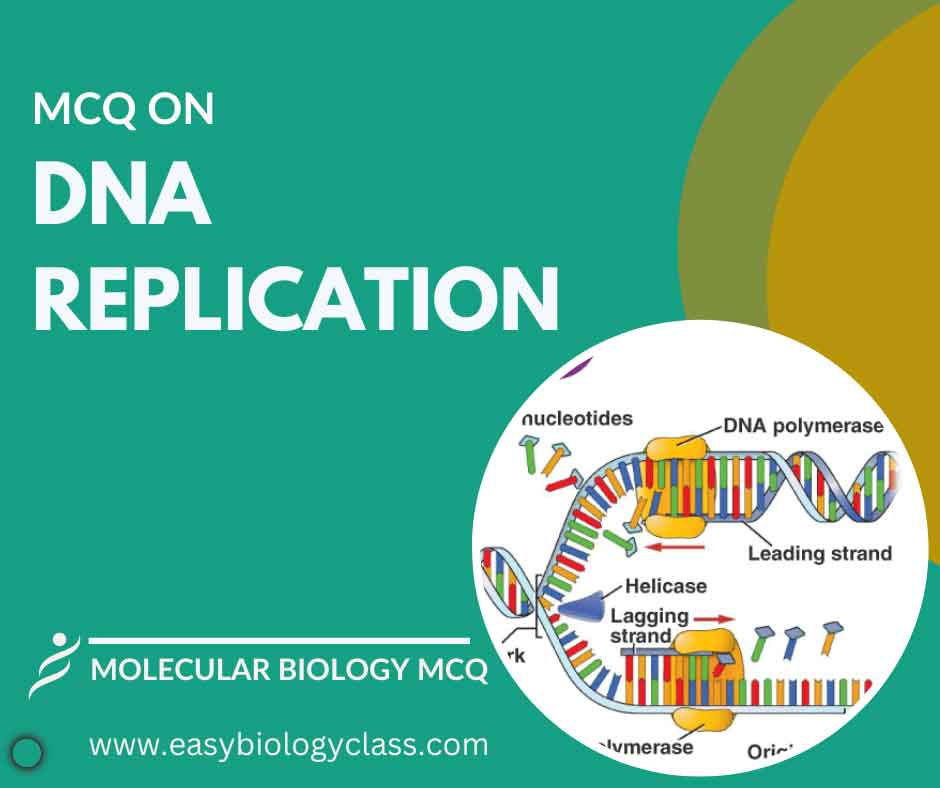
Continue ReadingMCQ on DNA Replication
DNA replication is the process by which a cell duplicates its DNA before cell division. It involves unwinding the double helix, complementary base pairing, and enzyme-mediated synthesis of new strands, ensuring genetic continuity and transmission of genetic information to daughter cells. This is an MCQ on DNA Replication in Prokaryotes […]
Continue Reading