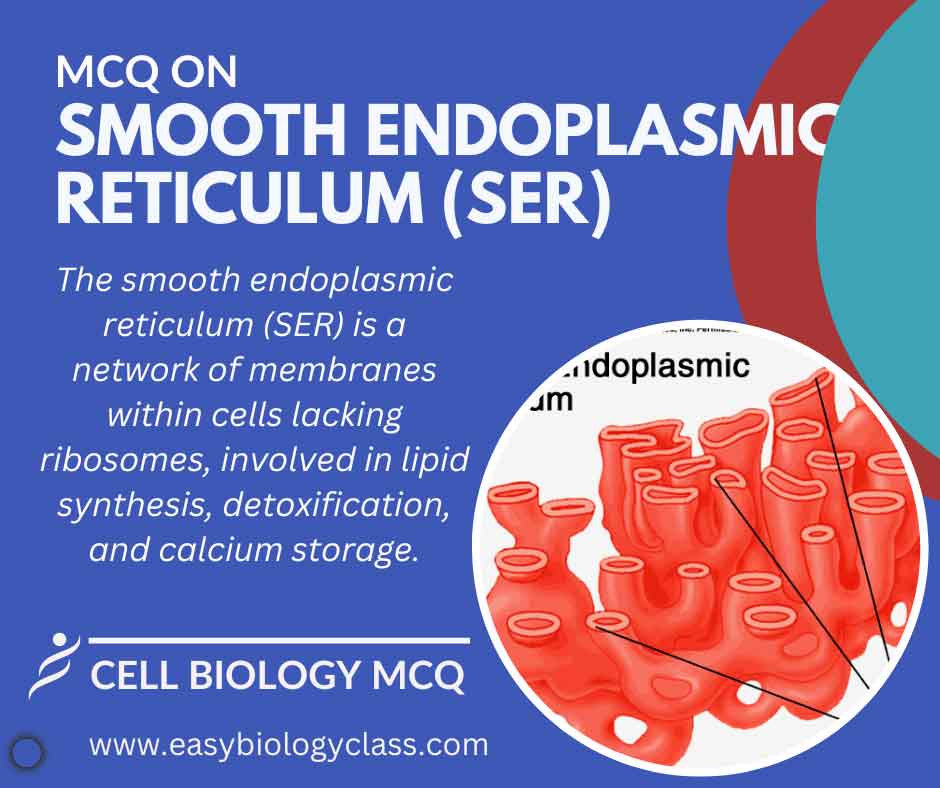
The smooth endoplasmic reticulum (SER) lacks ribosomes on its surface and is involved in lipid synthesis, detoxification, and calcium storage within eukaryotic cells. It plays crucial roles in various cellular processes, including metabolism, steroid hormone production, and drug metabolism. This is an MCQ on Smooth Endoplasmic Reticulum Structure and Functions. […]
Continue ReadingMCQ on Protein Function
MCQ on Protein Function: Proteins serve diverse roles in living organisms, including enzymatic catalysis, structural support, transport of molecules, immune defense, and cell signaling. They contribute to the regulation of gene expression, muscle contraction, and overall cellular function, essential for life processes. Biochemistry Notes | Biochemistry PPTs | Biochemistry MCQs […]
Continue ReadingMCQ on Functions of Lipids
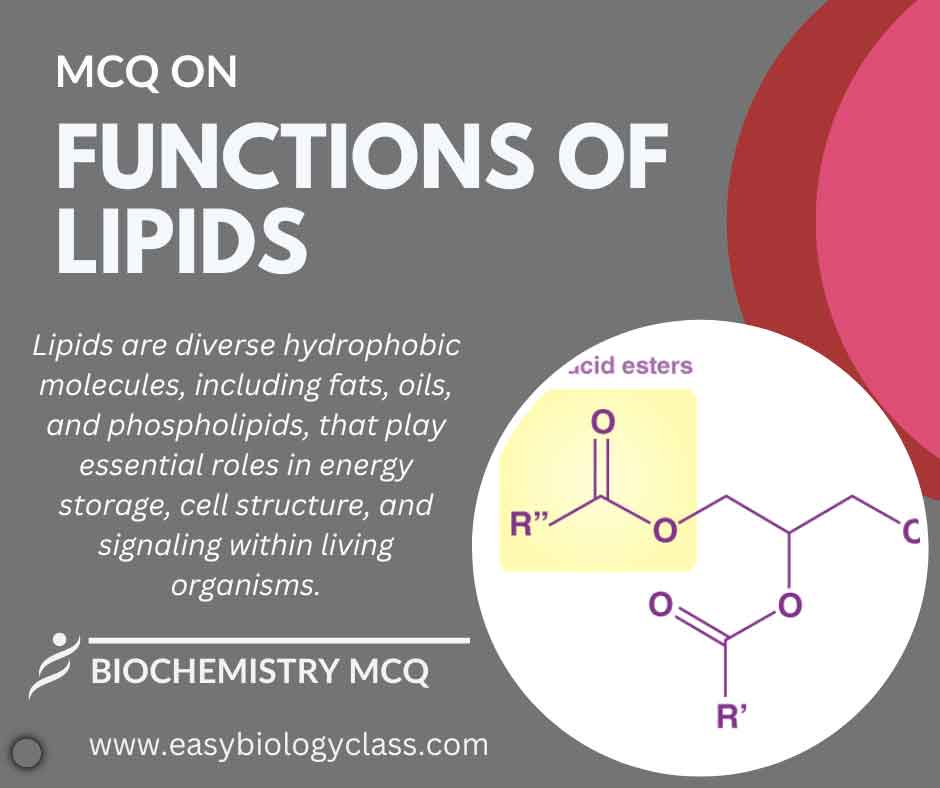
Lipids serve diverse functions, including energy storage, insulation, and cushioning. They form structural components of cell membranes, contribute to signaling molecules, and aid in nutrient absorption. Essential in various physiological processes, lipids play crucial roles in maintaining cellular integrity and supporting overall health. This is an MCQ on Functions of […]
Continue ReadingMCQ on Functions of Fatty Acids
Fatty acids serve vital roles in the body, functioning as energy sources, structural components of cell membranes, and precursors to signaling molecules. Essential fatty acids play key roles in maintaining cellular function, supporting brain health, and contributing to overall metabolic processes. This is an MCQ on Functions of Fatty Acids. […]
Continue ReadingMCQ on Functions of Enzymes
Enzymes are biological catalysts that accelerate chemical reactions in living organisms. They facilitate processes such as metabolism, DNA replication, and digestion by lowering activation energy. Highly specific, enzymes play crucial roles in maintaining cellular functions and enabling life-sustaining biochemical reactions. This is an MCQ on Functions of Enzymes. Biochemistry Notes […]
Continue Reading