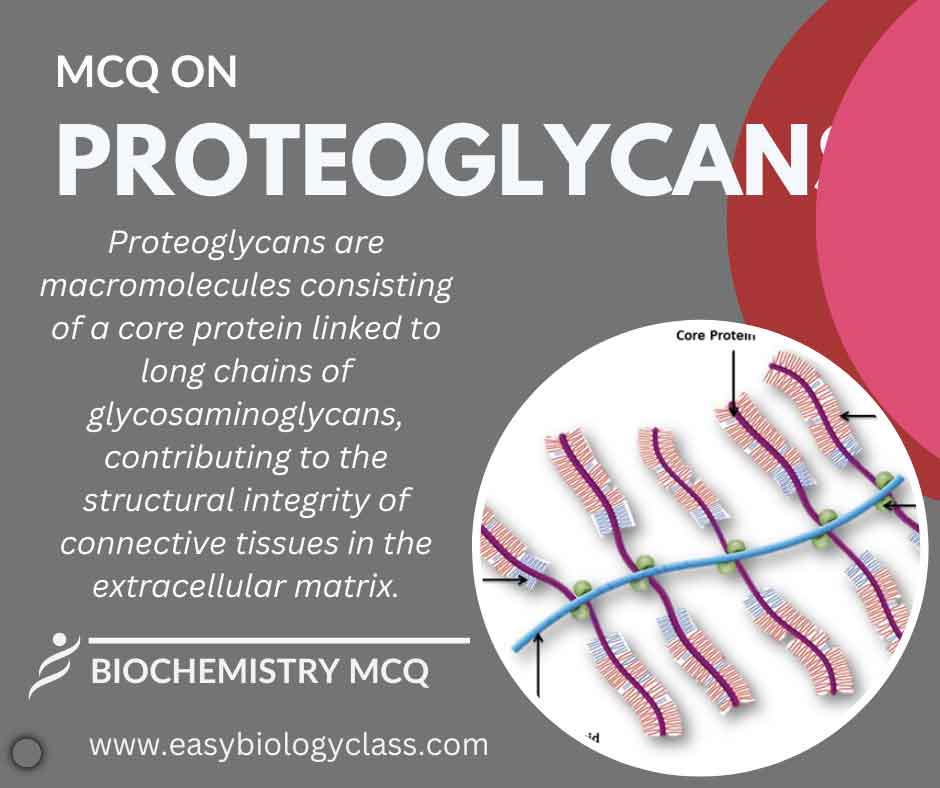
Proteoglycans are macromolecules composed of a core protein linked to long chains of glycosaminoglycans (GAGs). Abundant in extracellular matrices, they provide structural support, regulate cell behavior, and contribute to tissue resilience. The negatively charged GAGs confer unique properties to proteoglycans, influencing their biological functions. This is an MCQ on Proteoglycans […]
Continue ReadingTag Archives: Carbohydrates
MCQ on Carbohydrates Structure and Functions
Carbohydrates are organic compounds composed of carbon, hydrogen, and oxygen, typically in a 1:2:1 ratio. They serve as a primary source of energy for living organisms and include sugars, starches, and fibers found in foods like fruits, grains, and vegetables. This is an MCQ on Carbohydrates Structure and Functions. | […]
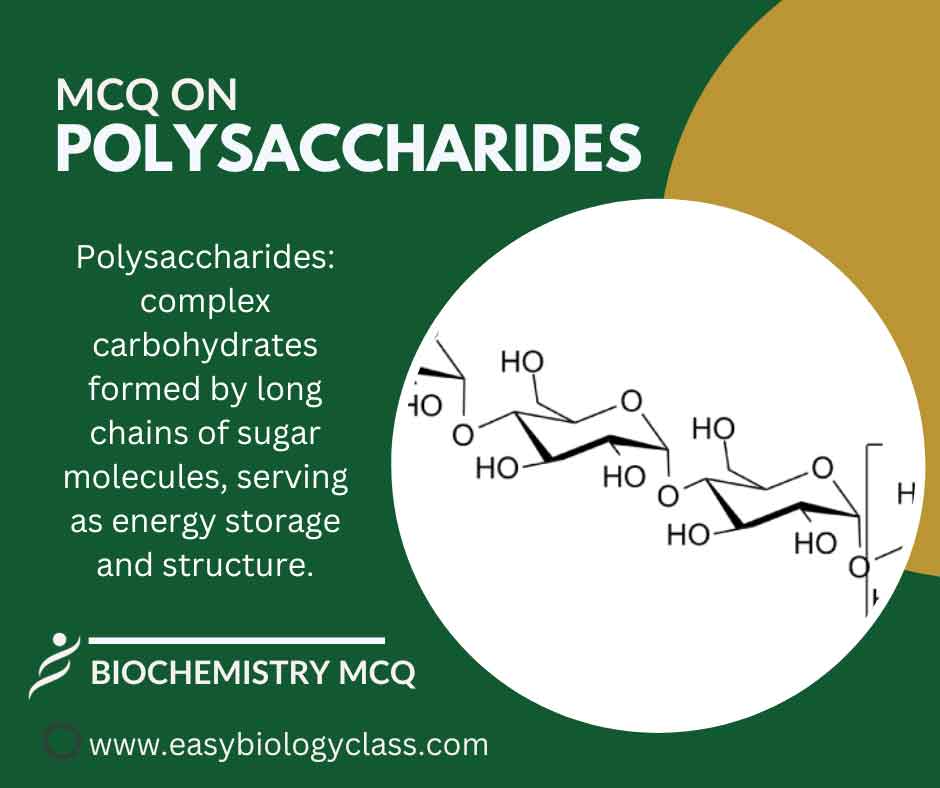
Continue ReadingMCQ on Polysaccharides
Polysaccharides are complex carbohydrates composed of multiple monosaccharide units linked together. Examples include starch, cellulose, and glycogen. They serve as energy storage (like glycogen) and structural components (like cellulose in plant cell walls) in living organisms. This is an MCQ on Polysaccharides Structure, Functions with Examples. Biochemistry Notes | Biochemistry […]
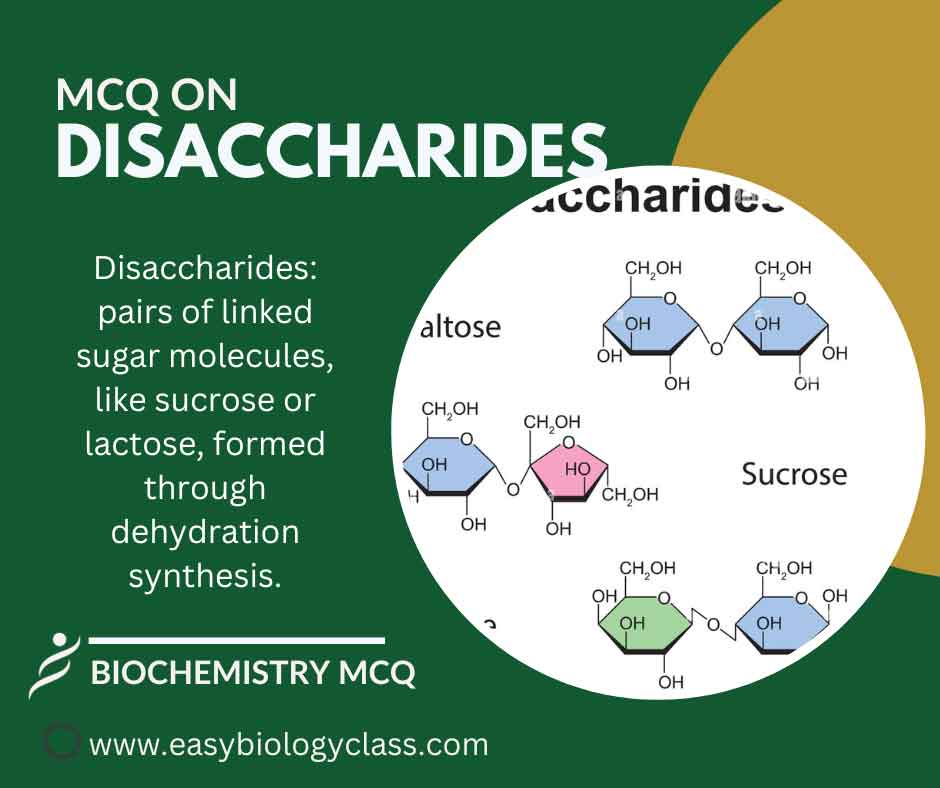
Continue ReadingMCQ on Disaccharides
Disaccharides are sugar molecules composed of two monosaccharides linked together by a glycosidic bond. Common examples include sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (glucose + glucose), serving as energy sources in food. This is an MCQ on Disaccharides: Structure, Functions and Examples Biochemistry Notes | Biochemistry […]
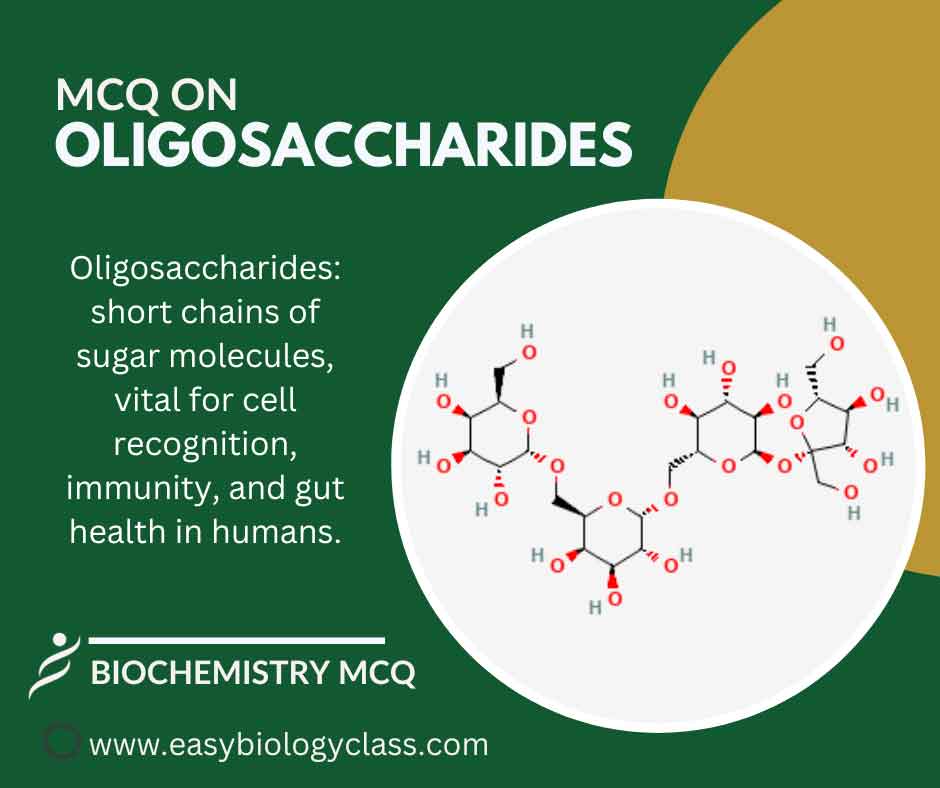
Continue ReadingMCQ on Oligosaccharides
Oligosaccharides are short chains of 3 to 10 linked sugar molecules, like maltose and sucrose. They’re intermediate between monosaccharides and polysaccharides, playing roles in cell recognition, immunity, and gut health as prebiotics or in cellular signaling. This is an MCQ on Oligosaccharides Structure, Functions and Examples. Biochemistry Notes | Biochemistry […]
Continue Reading