The pH scale is a measure of the acidity or alkalinity of a substance, ranging from 0 (highly acidic) to 14 (highly alkaline), with 7 being neutral. It quantifies the concentration of hydrogen ions in a solution, where lower values indicate higher acidity and higher values indicate greater alkalinity. This […]
Continue ReadingTag Archives: pH Scale
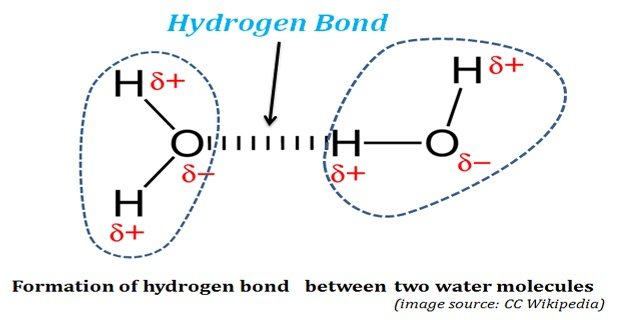
How Hydrogen Bond is Formed in Water?
Hydrogen Bonds in Water: The life was originated and started its evolution in water. Without water, life could not have existed on this planet. The properties of water, both physical and chemical, enabled water as the ‘solvent of life’. The water possesses some unusual physical and chemical properties. These ‘unusual […]
Continue ReadingWhy the pH of water is 7?
What is pH? ⊗. pH is defined as the negative logarithm of hydrogen ion concentration in a medium. ⊗. For example, if you are saying a solution with pH 6.5, it means that the concentration of H+ ions in that solution expressed in negative logarithmic term is 6.5. ⊗. pH […]
Continue Reading