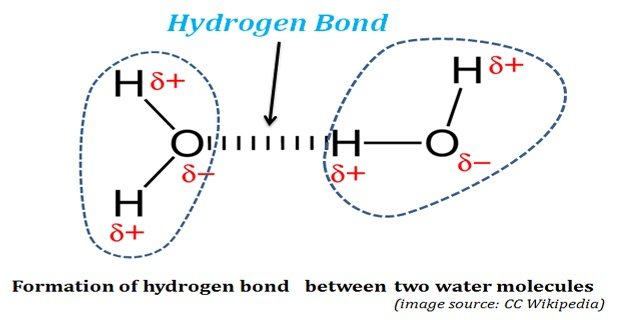
Hydrogen Bonds in Water: The life was originated and started its evolution in water. Without water, life could not have existed on this planet. The properties of water, both physical and chemical, enabled water as the ‘solvent of life’. The water possesses some unusual physical and chemical properties. These ‘unusual […]
Continue Reading