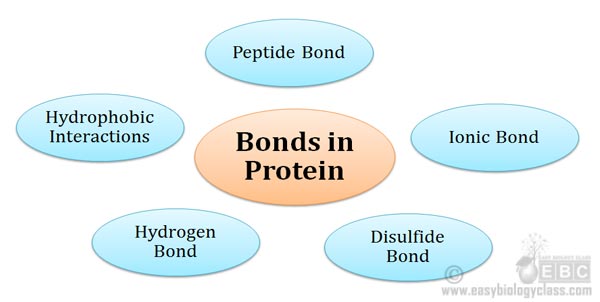
Bonds involved in Protein Structure Proteins are the polymers of amino acids. Amino acids are joined together by a special type of covalent bond (peptide bond) to form linear structures called polypeptides. The polypeptides are then folded into specific structures to form the functional conformation of the protein. The folding […]
Continue ReadingTag Archives: Biochemistry Tutorials
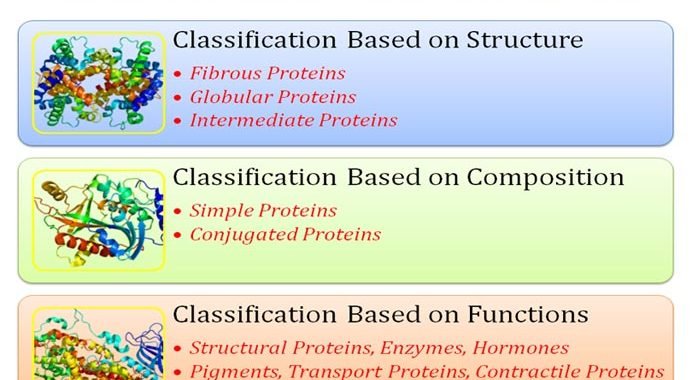
Classification of Proteins Based on Structure and Function
Classification of proteins: Proteins are important macromolecules of the cells, formed by the polymerization of amino acids according to the sequence of genetic code in the mRNA. Proteins are the mode of expression of the genetic information. They perform a variety of duties in the cells such as they act […]
Continue ReadingBiological Importance of Water
Biological Importance of Water Biological importance of water: Water is the mother liquid of all forms of life. The essentiality of water for living systems is quite evident as without water, there is no life. No other substance on earth is abundant as water. All aspects of cell structure and […]
Continue ReadingPhysical and Chemical Properties of Water and its Biological Significance
Physical and Chemical Properties of Water: Water is the most abundant substance in the living system. Water makes up about 70% or more of the weight of almost all organisms. The life has originated in remote past in the aqueous environment. The properties (both physical and chemical) of water enabled […]
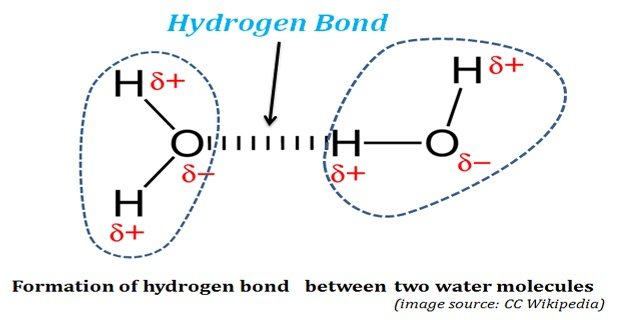
Continue ReadingHow Hydrogen Bond is Formed in Water?
Hydrogen Bonds in Water: The life was originated and started its evolution in water. Without water, life could not have existed on this planet. The properties of water, both physical and chemical, enabled water as the ‘solvent of life’. The water possesses some unusual physical and chemical properties. These ‘unusual […]
Continue Reading