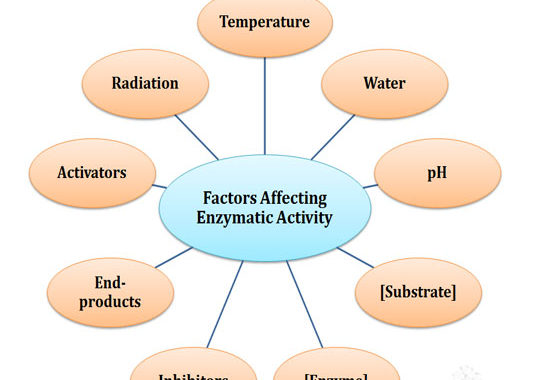
In the previous post, we have discussed the Properties of Enzymes. In the present post, we will see what all are the factors that affect the catalytic activity of an enzyme in the living system. Factors Affecting Enzymatic Activity Ø The catalytic activities of enzymes are affected by multiple factors. […]
Continue ReadingTag Archives: Biochemistry Short Notes
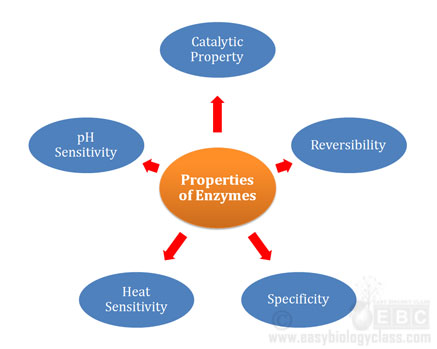
Properties of Enzymes (Biochemistry Lecture Notes)
Enzymes are biological catalysis. They are specialized proteins (except ribozymes) capable of catalyzing specific reactions in the cells. In the previous post, we have discussed the Structure and Functions of Enzymes. In the present post, we will discuss the Properties of Enzymes. What are the Properties of Enzymes? @. The […]
Continue ReadingIntroduction to Enzymes: Structure and Characteristics-Short Lecture Notes
What are Enzymes? There are thousands of chemical reactions in a living system. The chemical reactions in the cell are catalyzed by the biological catalysts called enzymes. Almost all enzymes are highly specialized proteins. (Exception: Ribozymes –Ribozymes are RNA with catalytic activity). The current post we will discuss the Characteristics […]
Continue ReadingTitration Curve of a Weak Acid and its pKa (Biochemistry Notes)
What is Titration? Titration is a method to determine the concentration of a dissolved substance (analyte or titrand) in a known volume by reacting it with another substance of known concentration and volume (titrant). The volume of the reactants plays a crucial role in the titration and thus the titration […]
Continue ReadingProton Hopping in Water (Grotthuss Mechanism)
Proton Hopping in water is the process of diffusion of protons (H⁺ ions) through the network of hydrogen-bonded water molecules in the liquid water. Proton hopping is also called as Grotthuss mechanism, named after the discoverer Theodor Grotthuss. The net result of proton hopping is the fast movement of H⁺ […]
Continue Reading