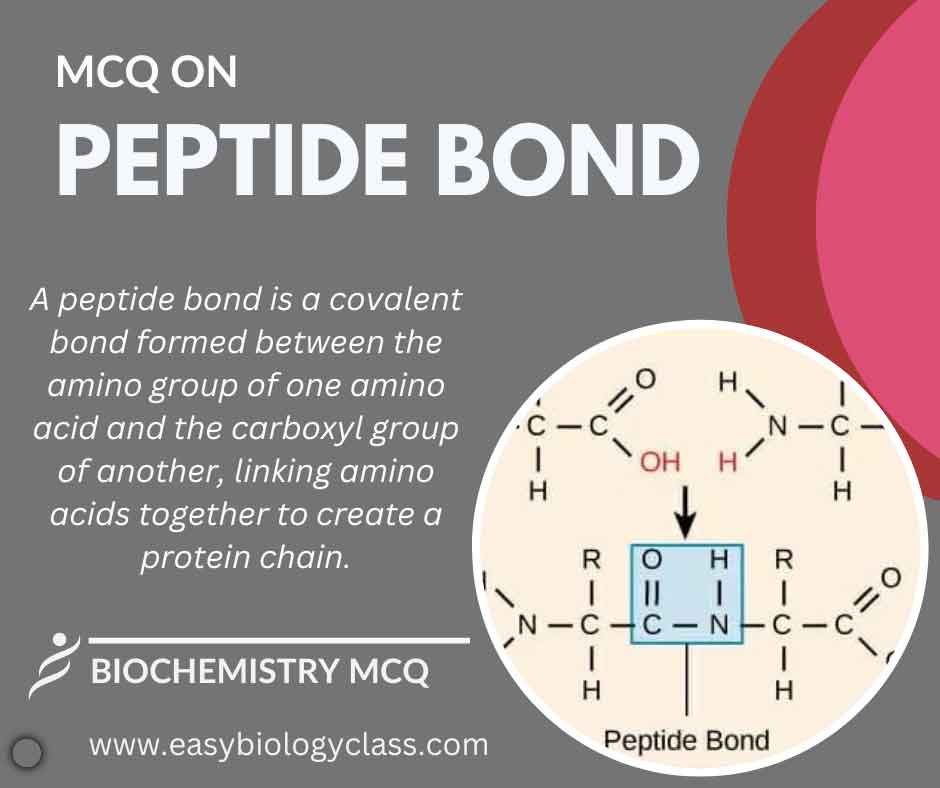
A peptide bond is a covalent linkage between the carboxyl group of one amino acid and the amino group of another. Formed during protein synthesis through dehydration synthesis, it creates a polypeptide chain, crucial for the structure and function of proteins in living organisms. This is an MCQ on Peptide […]
Continue ReadingMCQ on Proteoglycans
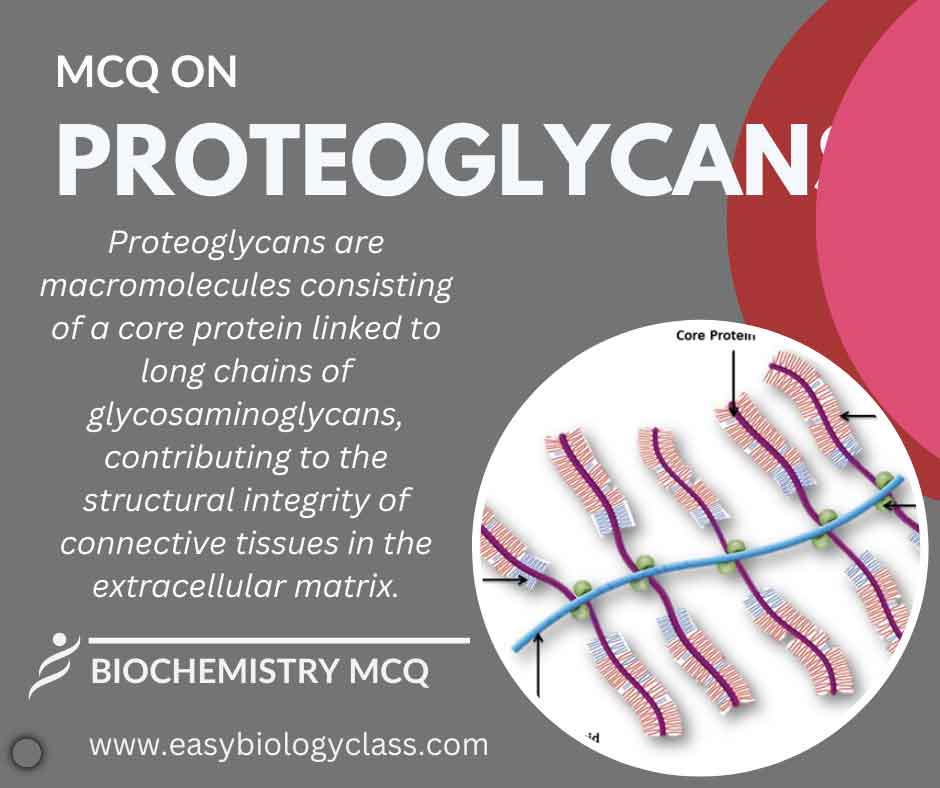
Proteoglycans are macromolecules composed of a core protein linked to long chains of glycosaminoglycans (GAGs). Abundant in extracellular matrices, they provide structural support, regulate cell behavior, and contribute to tissue resilience. The negatively charged GAGs confer unique properties to proteoglycans, influencing their biological functions. This is an MCQ on Proteoglycans […]
Continue ReadingMCQ on Comparative Genomics
Comparative genomics is the study of genetic similarities and differences among different species. It involves analyzing genome structures, gene content, and evolutionary relationships to understand the functional significance of genes and regulatory elements, aiding in diverse fields like evolutionary biology, medicine, and agriculture. This is an MCQ on Comparative Genomics. […]
Continue ReadingMCQ on Functional Genomics
Functional genomics is a field that explores the roles and interactions of genes within a genome. It involves studying gene functions, expression patterns, and regulatory mechanisms, utilizing various techniques to understand how genes contribute to biological processes and phenotypes. This is an MCQ on Functional Genomics. Bioinformatics Notes | Bioinformatics […]
Continue ReadingMCQ on Structural Genomics
Structural genomics is a field that aims to determine the three-dimensional structures of all possible proteins encoded by a genome. It employs high-throughput methods like X-ray crystallography and NMR spectroscopy to analyze protein structures, aiding in understanding their functions and supporting drug discovery efforts. This is an MCQ on Structural […]
Continue Reading