Monosaccharides are Simplest Sugars
Monosaccharides are the simplest carbohydrates. They are polyhydroxy aldehydes or ketones with a carbon backbone. The carbon backbone in monosaccharides usually consists of 3 – 6 carbon atoms. The simplest monosaccharides are glyceraldehyde and dihydroxyacetone (with 3 carbons). The most abundant monosaccharide in nature is a 6 carbon sugar called glucose. Majority of the monosaccharides follow the empirical formula C(H2O)n.
Monosaccharide with five or more carbon can predominantly exist as cyclic structures in the aqueous condition. All monosaccharides are colourless, crystalline solids and that are readily soluble in water but insoluble in nonpolar solvents. Most of the monosaccharides are sweet in taste.
More in Biochemistry: Lecture Notes, MCQ, PPTs, Videos
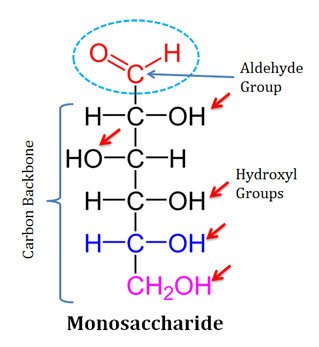 Chemical Structure of Monosaccharides
Chemical Structure of Monosaccharides
Ø All monosaccharides are polyhydroxy (contain many hydroxyl groups) aldehydes or ketones.
Ø The hydroxyl groups are attached to the carbon backbone.
Ø The number of carbon atoms in the backbone of monosaccharides varies from 3 to 6.
Ø The carbon backbone of monosaccharides is unbranched and individual carbon atoms are connected by single bonds.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Ø Monosaccharides are broadly classified into Aldoses and Ketoses.
Ø In the open chain conformation of a monosaccharide, one of the carbon atoms of the backbone is double bonded to an oxygen atom to form the carbonyl group (C=O).
Ø If the carbonyl group is at the end of the carbon chain it will be an aldehyde group (R – COH) and thus the sugar formed will be an Aldose sugar.
Ø Similarly, if the carbonyl group is inner to the carbon chain, it will be a keto group (C=O) and the sugar formed will be a Ketose sugar. 
Ø Based on the number of carbon atoms in the backbone, aldose sugars can be aldotriose (3C), aldotetrose (4C), aldopentose (5C) or aldohexose (6C).
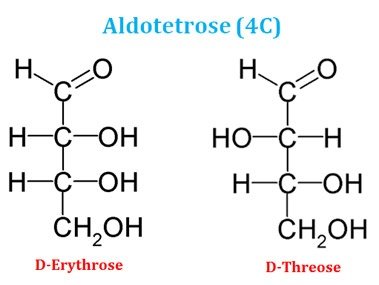
Examples of Aldotetrose (4 carbon sugars)
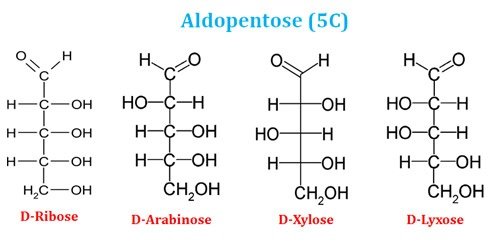
Examples of Aldopentose (5C sugars)
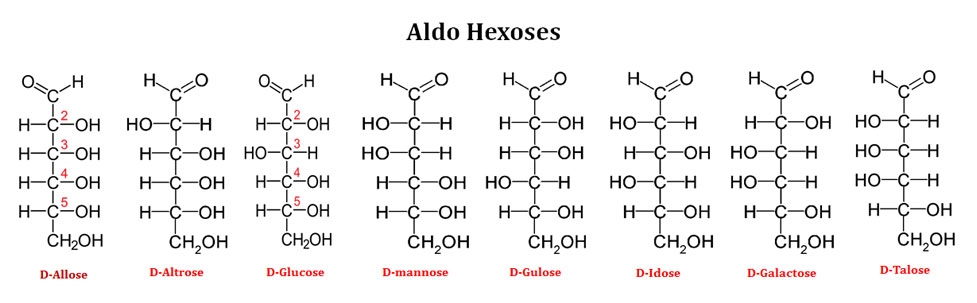
Examples of Aldohexoses (6C sugars)
Ø Similarly, based on the number of carbon atoms in the ketose sugars, they may be ketotriose (3C), ketotetrose (4C), ketopentose (5C) and ketohexose (6C).
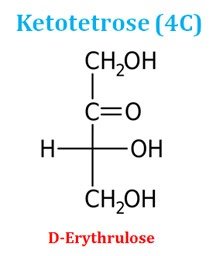
Ketotetrose (4C sugar)
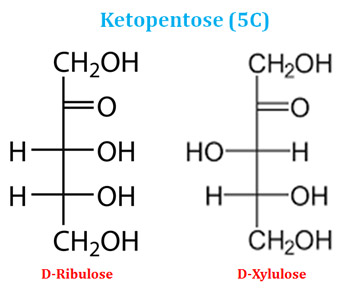
Ketopentose Examples (5C Ketose sugars)
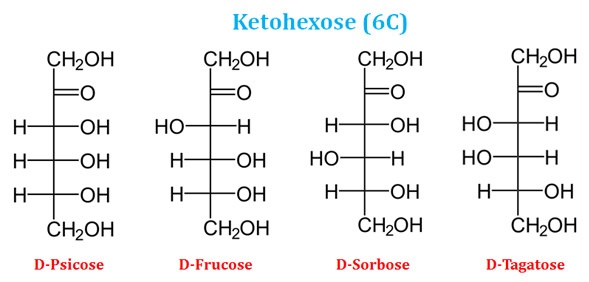
Ketohexose Examples (6C Keto Sugars)
Ø The simplest sugar in nature is with three carbons. (Example: Glyceraldehyde and Dihydroxyacetone).
Ø Glyceraldehyde is an aldotriose (simplest aldose sugar with 3C).
Ø Dihydroxyacetone is a ketotriose (simplest ketose sugar with 3C).

Ø Hexose sugars (with 6C) are the most abundant monosaccharides in the nature. Example: Glucose (an aldohexose) and Fructose (ketohexose)
Monosaccharides have asymmetric centers:
Ø All monosaccharides except Dihydroxyacetone contain one or more asymmetric centers (chiral centers).
Ø Due to the presence of the chiral centers, all monosaccharides (except Dihydroxyacetone) are optically active and can exist in many stereo-isomeric forms.
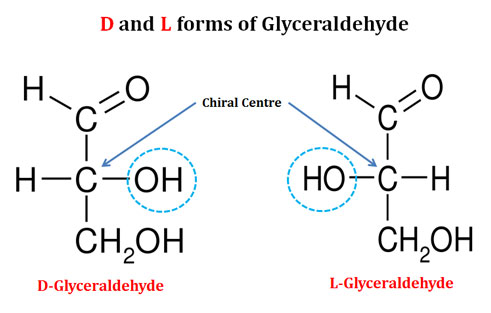
Ø The simplest monosaccharide – Glyceraldehyde contain one chiral center and thus it produces two optical isomers.
Ø These two optical isomers are designated as D-isomer and L-isomer.
Ø The D and L-isomers of a monosaccharide are together called as Enantiomers.
Ø For representing the D and L isomers of a monosaccharide in the paper, we commonly use the Fischer Projection Formula (as in the diagram).
Ø A monosaccharide with ‘n’ chiral centers can have 2n stereoisomers.
Ø Example:
@ Glyceraldehyde is with one chiral center, and thus it produces 21 = 2 isomers.
@ Glucose is with 4 chiral centers, and thus it produces 24 = 16 stereoisomers.
Ø In classical biochemistry, the stereoisomers of a monosaccharide are divided into two categories based on the configuration of the most distant chiral center (form carbonyl carbon).
Ø Those isomers with the configuration at this reference carbon similar to that of the D-glyceraldehyde are designated as D-isomers.
Ø Similarly, those with configuration at this reference carbon similar to that of L-glyceraldehyde are designated as L-isomers.
Ø By convention, if the hydroxyl group on the reference point carbon is on the right side in the Fischer projection formula, the sugar is a D-isomer (D-sugar).
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
Ø Similarly, if the hydroxyl group is on the left side, the sugar is designated as L-isomer (L-sugar).
Ø Among the total stereoisomers of a monosaccharide half of them will be D-isomers and the rest will be L-isomers.
Ø Example:
@ In the simplest sugar glyceraldehyde, among the two isomers, one is D form and the other is L form.
@ Similarly, aldohexose possesses 16 stereoisomers, among these 8 will be D forms and the remaining 8 will be L forms.
Ø Most of the naturally occurring hexoses in nature are D isomers.
Ø L-isomers of some sugars also occur in nature.
Ø Example: L-arabinose is a naturally occurring monosaccharide.
Ø L-isomers also present in the sugar derivatives and glycoconjugates.
Ø The carbon atoms in the monosaccharide are numbered from the end near to the carbonyl carbon.
Ø Each of the eight D-aldohexoses which differs in the stereochemistry at C2, C3, C4 or C5 has its own names.
Ø For example, in the case of aldohexose, they are named as Allose, Altrose, Glucose, Mannose, Gulose, Idose, Galactose and Talose.
Ø All these can exist in both D-forms and L-forms; however, the D-forms predominate in nature.
Ø Among the above mentioned eight forms of aldohexoses, D-glucose, D-mannose and D-galactose are common in nature. The rest are rare or can be produced artificially.
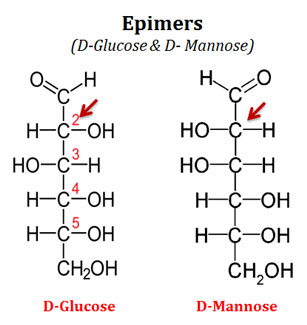
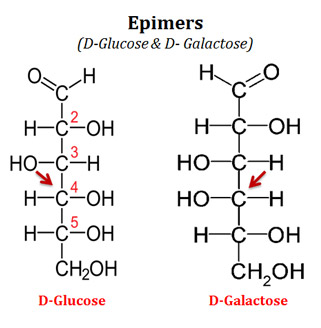 What are Epimers?
What are Epimers?
Ø Two sugars (a sugar pair) that differ only in the configuration around one carbon atom are called epimers.
Ø Example
@ D-glucose and D-mannose are epimers. They differ only at the stereochemistry of C2.
@ Similarly, D-glucose and D-galactose are epimers. They differ around the stereochemistry of C4.
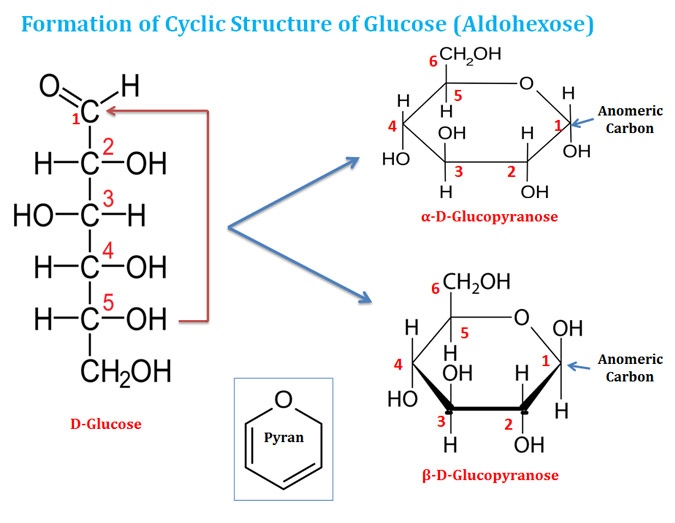
Cyclic structure of Monosaccharides
Ø Monosaccharides with five or more carbon atoms predominantly occur in cyclic form in the aqueous condition.
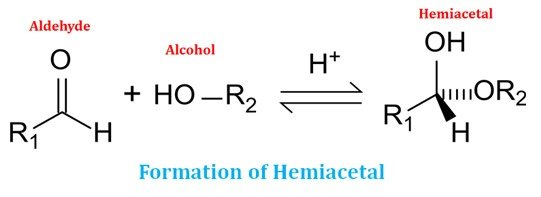
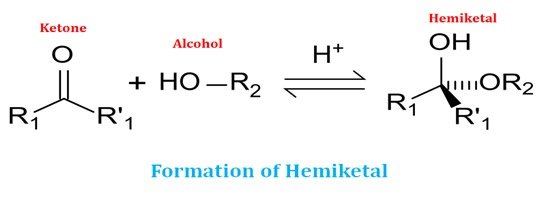
Ø For the formation of a cyclic structure, the carbonyl group forms a covalent bond with the oxygen atom of the hydroxyl group of the chain.
Ø The ring structure formation is due to the reaction of an alcohol (hydroxyl group) and an aldehyde group (in aldose sugar) or a keto group (in ketose sugar).
Ø The reaction between an alcohol and an aldehyde group cause the formation of a hemiacetal group.
Ø Similarly, the reaction between an alcohol and keto group results in the formation of a hemiketal group.
Ø The formation of cyclic structure in the monosaccharide as a result of hemiacetal or hemiketal reaction creates an additional chiral center (see the diagram).
.
.
Ø Thus, with the formation of a new chiral center, the cyclic structure can also produce two stereoisomeric forms around the newly formed chiral center.
Ø These two newly created isomeres are designated as ‘α’ and ‘β’ forms.
Ø In the case of aldohexose, the six-membered ring compounds are called ‘pyranose’ since it resembles a six-membered ring compound called ‘pyran’.
Ø Aldohexose can also form five-membered ring structures.
Ø The five-membered ring structures are called ‘furanose’ since they resemble the structure of a five-membered ring compound called ‘furan’.
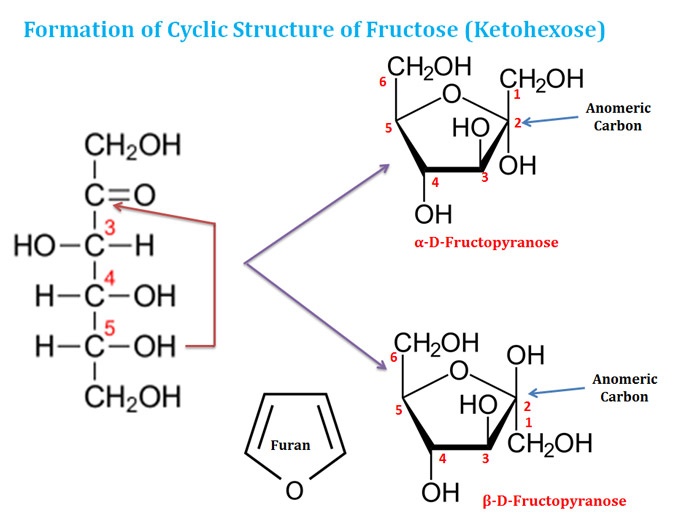
Ø Six-membered ring structures are more stable than five-membered rings and they are more abundant in nature.
Ø Isomeric forms of monosaccharides that differ only in their configuration at the hemiacetal or hemiketal carbon atoms are called Anomers. Example α-D-glucose and β-D-Glucose
Ø The hemiacetal or hemiketal carbon atom is called the anomeric carbon atom.
Ø The ‘α’ and ‘β’ anomers of glucose inter-convert in the aqueous solution by a process called mutarotation.
Ø Ketohexose can also form the ring structures.
Ø In ketohexose, the carbonyl O forms the covalent bond with the C5 (usually) or C6 (rarely) to form a furanose (common) or pyranose (rare) structure.
Ø The cyclic structures of monosaccharides are represented in a paper using the Haworth Perspective Formula.
Ø The cyclic structure of monosaccharides is not planar. They can assume either of the two ‘chair’ conformations.
Ø The two conformations are also interchangeable by the breakage and reunion of the covalent bonds.
All Monosaccharides are reducing Sugars
Ø All monosaccharides are reducing sugars.
Ø Sugar molecules which can reduce the ferric or cupric ions are called reducing sugars.
Ø Monosaccharides can be oxidized by mild oxidizing agents such as ferric (Fe3+) or cupric (Cu2+) ions.
Ø When the monosaccharides are treated with these reagents the carbonyl carbon (C=O) is oxidized to carboxyl group (COOH).
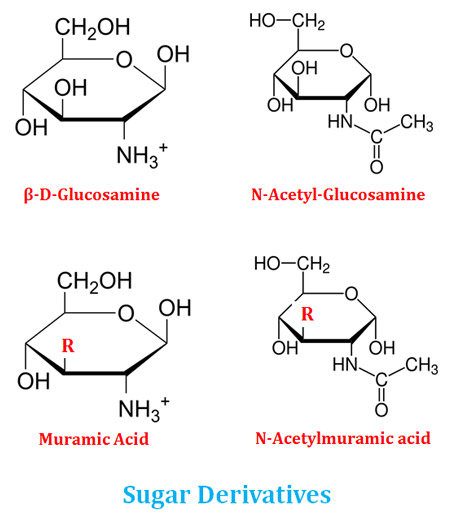
Sugar Derivatives of Monosaccharides
Ø Monosaccharides, particularly hexoses, can produce a wide variety of compounds called sugar derivatives by combining with other functional groups.
Ø In sugar derivatives, the hydroxyl groups of the carbon backbone are replaced by other functional groups or other compounds.
Ø Examples: Glucosamine, N-acetyl glucosamine, Muramic acid, N-acetyl muramic acid
Learn more: Sugar Derivatives
Functions of Monosaccharides
Ø Some monosaccharides such as glucose act as fuel for metabolic energy.
Ø Monosaccharides are the building blocks of complex sugars.
Ø Part of genetic material (ribose in RNA and deoxyribose in DNA).
Ø For the formation of sugar derivatives: example – glucosamine, muramic acid etc.
Review questions
- 1. Explain the structure of monosaccharides.
- 2. Differentiate aldoses from ketoses with examples
- 3. Explain the stereo-isomerism in monosaccharides with suitable diagrams.
- 4. What are epimers? Give examples
- 5. Differentiate the D and L forms of glyceraldehyde with diagram.
- 6. Explain the formation of cyclic structures in hexose sugars.
- 7. What are anomers? Give examples
- 8. Differentiate the alpha and beta forms of D-glucopyranose.
- 9. Why all monosaccharides are reducing sugars?
- 10. Write a short note on sugar derivatives with examples.
- 11. What are the functions of monosaccharides?
You might also like…
@. Carbohydrates Part 1: Introduction
@. Carbohydrates Part 2: Monosaccharides
@. Carbohydrates Part 3: Oligosaccharides / Disaccharides
@. Carbohydrates Part 4: Polysaccharides
@. Carbohydrates Part 5: Glyco-conjugates (Glycoproteins & Proteoglycans)
@. Carbohydrates Part 6: Glycosaminoglycans
@. Membrane Lipids: Properties, Structure and Classification
@. MCQ on Carbohydrates: Part 1 | Part 2 | Part 3 |
