(1). What is the molecular weight of water?
a. 10 g/mol
b. 15 g/mol
c. 18 g/mol
d. 20 g/mol
(2). What is the H+ ion concentration in pure water?
a. 1 X 10-7 m
b. 1 X 107 m
c. 1 X 10-14 m
d. 1 X 1014 m
Learn more: MCQ on pH | MCQ on Hydrogen Bonding
(3). The equilibrium constant of ionization reaction of pure water is:
a. 1.8 X 10-14 M
b. 1.8 X 10-16 M
c. 1.8 X 10-7 M
d. 1.8 X 10-7 M
(4). The pH of pure water is neutral, the best explanation for this is:
a. The pH of pure water is 7
b. In pure water the concentration of H+ and OH– are same
c. Water do not contain free H+ or OH– ions
d. What will never ionize
(5). What is the concentration of OH– ions in a solution with an H+ ion concentration of 1.3 X 10-4M?
a. 7.7 X 10-11 M
b. 7.7 X 10-10M
c. 1.4 X 10-11M
d. 1.4 X 10-10 M
(6). As the pKa of an acid increases, the acid will be:
a. More weaker
b. More stronger
c. Converted to neutral solution
d. Converted to basic solution
(7). Buffers are mixtures of:
a. Strong acid and strong base
b. Strong acid and weak base
c. Weak acid and their conjugate base
d. Weak base and their conjugate acid
(8). If a solution has to be a buffer, its pH should be
a. At its pKa value
b. At is Ka value
c. At 7
d. At 14
(9). The most important peculiarity of water when compared to other solvents is that water has:
a. High boiling point, high melting point and high heat of vaporization
b. High boiling point, low melting point and low heat of vaporization
c. Low boiling point, high melting point and low heat of vaporization
d. Low boiling point, low melting point and low heat of vaporization
(10). Which of the following is the correct representation of Henderson-Hasselbalch equation?
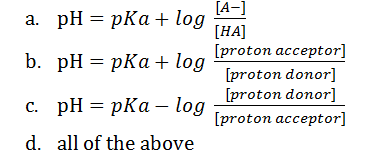
(11). According to Henderson-Hasselbalch equation, when the pH of a solution becomes equal to its pKa, the solution becomes a buffer. This condition is achieved when _____
a. The concentration of proton donor equals the concentration of proton acceptor
b. Concentration of proton donor become zero
c. Concentration of proton acceptor become zero
d. The concentration of proton donor become log 1/10th of concentration of proton acceptor
(12). Normal pH of blood is
a. 7.0
b. 7.2
c. 7.3
d. 7.4
(13). The heat of vaporization of water molecule at atmospheric temperature is:
a. 2260 J/g
b. 1260 J/g
c. 2260 k.cal
d. 1260 k.cal
(14). The water level in the human body is regulated by the hormone _____
a. ACTH
b. Oxytocin
c. FSH
d. Epinephrine
(15). The polarity of water molecule is due to
a. Difference in electronegativity of oxygen and hydrogen atoms in water
b. The readily ionizing behavior of water
c. The positive charge of water molecule
d. The negative charge of water molecule
(16). The van der Waals radius of hydrogen atom in water molecule is
a. 1.2 Å
b. 1.4 Å
c. 1.6 Å
d. 1.8 Å
Please take advantage of our Lecture Notes, PPTs, Previous Year Questions, Mock Tests
You may also like NOTES in...
BOTANY BIOCHEMISTRY MOL. BIOLOGY
ZOOLOGY MICROBIOLOGY BIOSTATISTICS
ECOLOGY IMMUNOLOGY BIOTECHNOLOGY
GENETICS EMBRYOLOGY PHYSIOLOGY
EVOLUTION BIOPHYSICS BIOINFORMATICS
Answer Key, Explanations and References
(1). Ans. (c). 18 g/mol
Exactly the molecular weight of water is 18.01528 g/mol
(2). Ans. (a). 1 X 10-7 m
(3). Ans. (b). 1.8 X 10-16 M
(4). Ans. (b). In pure water the concentration of H+ and OH- are same
(5). Ans. (a). 7.7 X 10-11 M
Use the equation of ion product of water
(6). Ans. (a). More weaker
pKa is the negative log of Ka. Strong acids will have higher Ka values where as weak acids will have lower Ka values.
(7). Ans. (c). Weak acid and their conjugate base
(8). Ans. (a). at its pKa value
(9). Ans. (a). High boiling point, high melting point and high heat of vaporization
(10). Ans. (d). all the above
(11). Ans. (a). The concentration of proton donor equals the concentration of proton acceptor
(12). Ans. (d). 7.4
(13). Ans. (a). 2260 J/g
(14). Ans. (b). Oxytocin
(15). Ans. (a). Difference in electronegativity of oxygen and hydrogen atoms in water
(16). Ans. (a). 1.2 Å
Reference: (1). Lehningers Principles of Biochemistry: Chapter: Water
(2). Fundamentals of Biochemistry by Voet and Voet: Chapter: Water
MCQ on Water, pH and Buffer | Part – 2 | Part – 1 |
You may also like...
NOTES QUESTION BANK COMPETITIVE EXAMS.
PPTs UNIVERSITY EXAMS DIFFERENCE BETWEEN..
MCQs PLUS ONE BIOLOGY NEWS & JOBS
MOCK TESTS PLUS TWO BIOLOGY PRACTICAL
