What are enzymes?
Ø All living organisms in the world should possess TWO fundamental properties
Ø They are:
(1). Must be able to self-replicate
(2). Must be able to catalyze chemical reactions efficiently and selectively
Ø Enzymes are the molecules which enables each living entity to do these two fundamental activities
Ø Enzymes are better known as biological catalysts
Ø Almost all enzymes are highly specialized proteins
Ø Ribozymes: RNA molecules with catalytic properties
Ø Abzymes: antibodies with catalytic properties
What is enzyme regulation?
Ø Enzyme regulation definition: “Process, by which cells can turn on, turn off, or modulate the activities of various metabolic pathways by regulating the activity of enzyme”
Ø Enzymes have extraordinary catalytic power
Ø They can increase the rate of chemical reaction thousand fold
Ø At optimum conditions enzymes are able to convert millions of substrate molecules into products in a short fraction of time
Ø It is very essential to control the activity of enzyme in order to regulate the metabolic activities in the cell
Ø Enzyme regulation will permit the changing needs of the cell to meet its energy and resource demands
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Ø If a product is available in excess, enzyme regulation could then divert the resources to other needy reactions
Ø If a product is in demand, it could activate pathways to produce more of the biomolecule that is needed
What are regulatory enzymes?
Ø In cellular metabolic activities, many enzymes work together in a sequence to carry out the given metabolic process
Ø Example, glycolysis pathway includes ten sequential steps each catalyzed by specific enzymes
Ø In such an enzymatic process, were more than one steps are involved, the reaction product of one enzymatic reaction acts as the substrate for the next enzyme
 Ø Regulatory enzyme definition: “In a multi-step enzymatic process, there will be one enzyme which will be responsible for the overall rate of that process”
Ø Regulatory enzyme definition: “In a multi-step enzymatic process, there will be one enzyme which will be responsible for the overall rate of that process”
Ø This critical rate limiting enzyme is called the regulatory enzyme
Ø Regulatory enzyme shows enhanced or decreased catalytic activities in response to other molecules (signals) in the cells
Which enzyme is the regulatory enzyme in a multi-step metabolic pathway?
Ø First enzyme of multi-enzyme metabolic sequence acts as the regulatory enzyme
Ø First reaction is the excellent place to regulate or control a metabolic pathway
Ø This is because catalysis of even the first few reactions consumes much metabolic energy which can be diverted to other more important processes
Mechanisms of enzyme regulation:
Ø FIVE different types of enzymatic regulation mechanism occurs in the cells
Ø Activities of the regulatory enzyme is modulated in a variety of ways
Ø Different types of enzyme regulation methods are:
(1). Allosteric enzymes (Allosteric regulation of enzymes)
Feedback inhibition
(2). Reversible covalent modification of enzymes
(3). Proteolytic activation of enzyme
(4). Feedback regulation
(5). Regulation by Isoenzymes (isozymes)
(1). Allosteric enzymes
What is allosteric regulation of enzyme?
Ø Allosteric enzymes are a class of regulatory enzymes
Ø Allosteric regulation definition: A type of enzyme regulation by the reversible non-covalent binding of regulatory molecules to the enzyme
Ø Regulatory molecules are called allosteric modulators or allosteric effectors
Ø Allosteric enzymes have additional conformations induced by the binding of modulators
Ø Conformational changes induced by the allosteric modulators can produce more active or less active forms of enzyme
Ø Allosteric modulators may be inhibitory (+) or stimulatory (-)
Ø Two types of Allosteric enzymes based on the nature of modulator:
1. Homotropic allosteric enzymes
2. Heterotropic allosteric enzymes
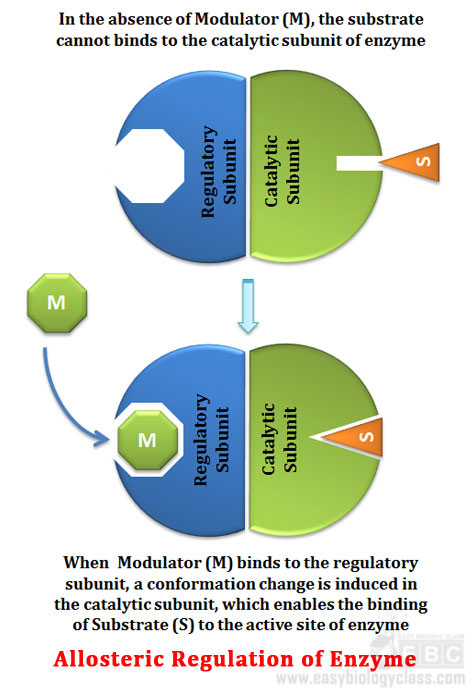 Ø In most cases, the substrate itself acts as the modulator
Ø In most cases, the substrate itself acts as the modulator
Ø Allosteric enzymes having the substrate and modulators are same are called homotropic allosteric enzymes
Ø Binding of modulator causes conformational changes in the enzyme
Ø Conformational changes affect the subsequent enzymatic activity
Ø If modulator is any molecule other than substrate, the enzyme is called heterotropic allosteric enzyme
Ø Allosteric modulators are not to be considered as competitive or non-competitive inhibitors
Ø Allosteric enzymes possess one or more regulatory or allosteric sites
Ø Allosteric sites acts as the binding site of modulator
Ø Modulator and modulator binding site on enzyme are very specific similar to the substrate specific for its active site
Ø Allosteric enzymes are usually larger and more complex than non-allosteric enzymes
Ø Allosteric enzymes possess many sub-units
Ø Aspartate transcarbamoylase (an allosteric enzyme), which catalyze an early reaction in the biosynthesis of pyrimidine nucleotides, has 12 polypeptide chains organized into catalytic and regulatory sub-units. Enzymes with several modulators have different and specific binding sites for each
Ø In most of the allosteric enzymes, substrate binding side and modulator binding sites are on different subunits
Ø Substrate binding site is called catalytic subunit or C subunit
Ø Modulator binding subunit is called the regulatory subunit or R subunit
Ø Binding of a positive or stimulatory modulator (M) to its specific site on the regulatory subunit is communicated to the catalytic subunit through conformational changes
Ø This change renders the activation of catalytic subunit
Ø Activation enables the binding of substrate (S) with higher affinity
Ø Once the modulator is dissociate form the regulatory subunit, the enzyme reverts to its inactive or less active form
Ø Allosteric enzymes shows variations in enzyme kinetic parameters
Ø Allosteric enzymes do not follow Michaelis-Menten Kinetics
Ø Allosteric enzymes do not show the usual hyper-parabolic curve when the initial velocity Vo is plotted against substrate concentration [S]
Ø They shows a sigmoid curve when the velocity is plotted against substrate concentration
Ø The Lineweaver-Burk plot also shows difference from usual enzymes
Ø Lineweaver-Burk plot of an allosteric enzyme will be upward concave shaped
Ø Feedback inhibition is a type of allosteric regulation
What is Feedback inhibition?
Ø Feedback inhibition and feedback regulation are different terms
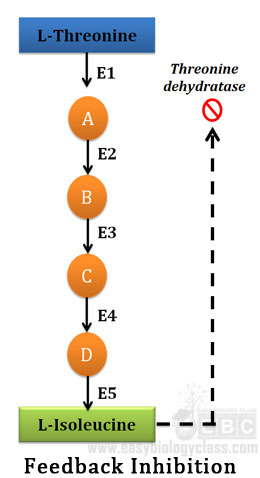 Ø Feedback inhibition is a specific type of allosteric enzymatic activity regulation mechanism in cells
Ø Feedback inhibition is a specific type of allosteric enzymatic activity regulation mechanism in cells
Ø Feedback inhibition definition: in some multi-enzyme pathways, the regulatory enzyme is specifically inhibited by the end product of the pathway whenever the concentration of the end product exceeds the cell’s requirements
Ø When the regulatory enzyme reaction is slowed, all subsequent enzymes operate at reduced rates as their substrates are also depleted
Ø Rate of production of the pathway’s end product is thereby brought into balance with the cell’s needs
Ø This type of regulation by the end product inhibiting to the first enzyme of the multi-enzymatic pathway is called feedback inhibition
Example for feedback inhibition:
Ø Most cited example of allosteric feedback inhibition is the biosynthesis of L-isoleucine from L-threonine in bacteria
Ø In bacteria the conversion of L-threonine to L-isoleucine occurs in five steps
Ø Each step is catalyzed by a specific enzyme
Ø First enzyme in this system is Threonie dehydratase
Ø Threonine dehydratase is inhibited by isoleucine, the product of the last reaction (end product)
Ø This is an example of heterotropic allosteric inhibition
Ø Isoleucine is quite specific as an inhibitor
Ø Any other intermediate in this sequence cannot inhibit threonine dehydratase
Ø Also, any other enzyme in the sequence is not inhibited by isoleucine
Ø Isoleucine binds not to the active site of the enzyme
Ø It binds to another specific site on the enzyme molecule, the regulatory site
Ø The binding is non-covalent and readily reversible
Ø When the isoleucine concentration decreases, the isoleucine binds to the regulatory site of threonine dehydratease detaches and making the enzyme active again
Ø Thus threonine dehydratase activity responds rapidly and reversibly to fluctuations in the cellular concentration of isoleucine
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
(2). Reverse covalent modification:
How reversible covalent modifications regulate enzymatic activity?
Ø Here, catalytic activity is modulated by reversible covalent modification of enzyme
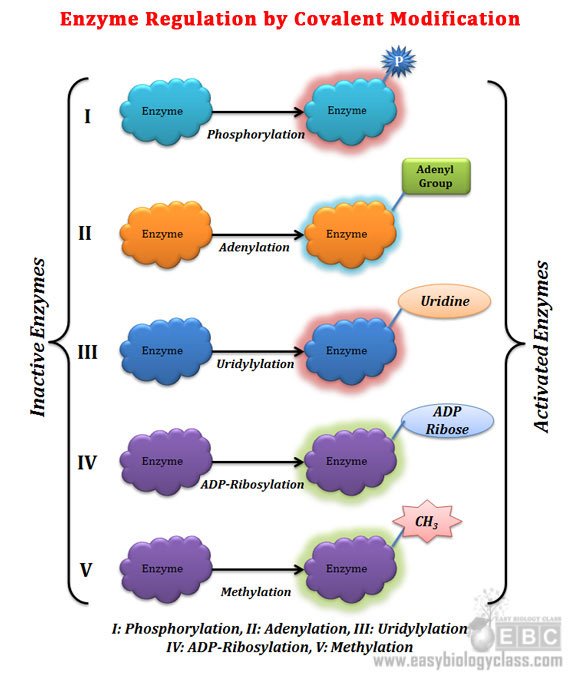 Ø More than 500 different molecules are known to modify enzymes by this method
Ø More than 500 different molecules are known to modify enzymes by this method
Ø Most common modifying groups include phosphoryl, acetyl, adenylyl, uridylyl, methyl, amide, carboxyl, prenyl, hydroxyl, sulfate, and adenosine diphosphate ribosyl groups
Ø There will be separate enzyme for adding and removing of modifying groups to enzymes
Ø Common reversible covalent modifications of enzyme:
@. Phosphorylation: most common type, addition of phosphate group to Tyr, Ser, Thr and His residue of protein
@. Adenylation: addition of adenine to Tyr residue of protein
@. ADP-ribosylation: addition of ADP ribose to Arg, Gln, Cys and Diphthamide of protein (dipthamide is a modified Histidine residue)
@. Methylation: addition of methyl group to Glu residue of protein
Ø ADP ribose derived from Nicotinamide Adenine Dinucleotide (NAD) is added to bacterial enzyme Dinitrogenase reductase resulting in the regulation of important process of biological nitrogen fixation
Ø Diphtheria toxin and cholera toxin are enzymes that catalyze the ADP ribosylation (and inactivation) of key cellular enzymes or proteins
 Ø Diphtheria toxin inhibits elongation factor 2 (EF2) of protein biosynthesis
Ø Diphtheria toxin inhibits elongation factor 2 (EF2) of protein biosynthesis
Ø Cholera toxin acts on a G protein that is part of a signaling pathway, leading to several physiological responses including a massive loss of body fluids and sometimes death
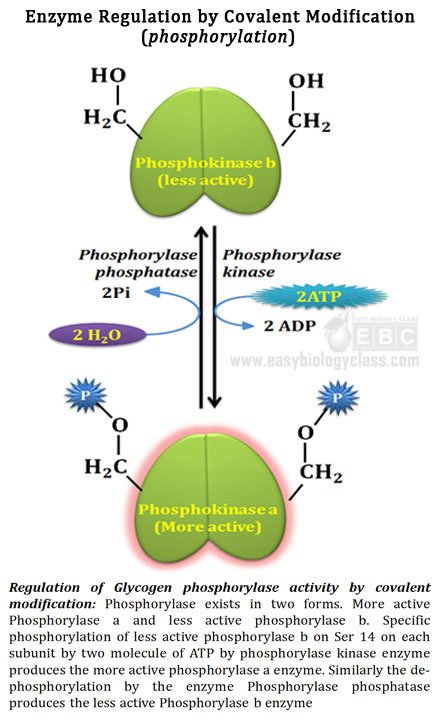
Ø Phosphorylation is the most common type of regulatory covalent modification
Ø Addition of phosphoryl groups to specific amino acid residues of a protein is called phosphorylation
Ø Phosphorylation reaction is catalyzed by the enzyme protein kinase.
Ø ATP or GTP usually acts as the phosphate group donor
Ø The energy derived from the cleavage of phosphate group donor is also utilized
Ø One third to one half of all proteins in a eukaryotic cells are phosphorylated
Ø Some proteins have only one phosphorylated residue, other have several sites for phosphorylation
Ø Phosphoryl groups are usually added to Ser, Thr or Thy residues of the enzyme
Ø Phosphorylation introduces a bulky charged group into a region that was only moderately polar
Ø Removal of phosphoryl group from a protein is called dephosphorylation
Ø Dephosphorylation reaction is catalyzed by protein phosphatase
(3). Proteolytic activation of enzymes:
How proteolytic cleavage of enzyme acts as a regulatory mechanism?
Ø Enzymes regulated by proteolytic cleavage method are produced first as inactive forms
Ø Inactive form of enzyme is called zymogen or pro-enzyme
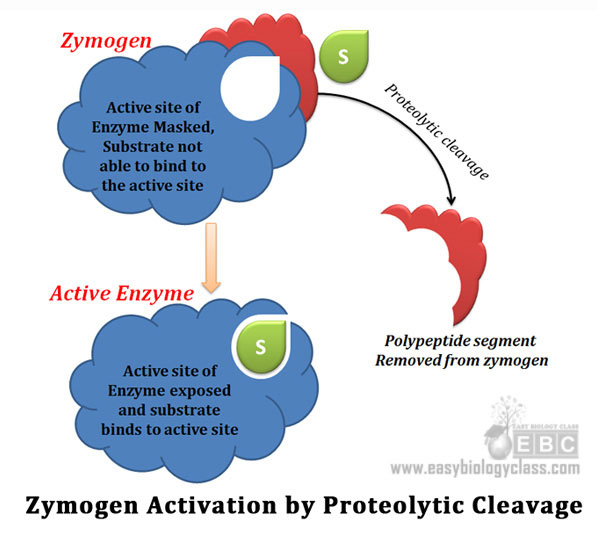 Ø Inactive nature of zymogens is duet the fact that the active site will be masked or covered by part of polypeptide chain
Ø Inactive nature of zymogens is duet the fact that the active site will be masked or covered by part of polypeptide chain
Ø Zymogens are later converted to active enzymes
Ø Conversion is done by the removal of specific parts of the enzyme by proteolytic cleavage
Ø Specific cleavage causes conformational changes that expose the active site of enzyme
Ø This type of activation is irreversible (once enzyme is activated, it cannot be made inactive)
Ø Proteolytic enzymes (proteases) of stomach and pancreas are regulated by proteolytic cleavage mechanism
Chymotrypsinogen → Chymotrypsin
Trypsinogen → Trypsin
Pepsinogen → Pepsin (autoactivation, enzyme activates itself)
Procarboxypeptidase → Carboxypeptidase
Proelastase → Elastase
Procollagen → Collagen
Procaspase → Caspase
Prothrombin → Thrombin
Fibrinogen → Fibrin
Ø Significance of enzyme production as zymogen:
@. Helps to prevent the autocatalytic damage of cellular components
@. Assist in the mobilization of enzyme in the cell
@. Can be converted to active forms when it is needed
@. Can be stored for long time as zymogen
@. Hal-life of zymogens are usually more than its active enzymes
(4). Feedback regulation
What is Feedback regulation?
Ø Feedback regulation is different from feedback inhibition
Ø A type of enzymatic activity regulation
Ø Here the end product of an enzymatic pathway directly inhibit the synthesis of concerned enzyme by interfering with the gene of that enzyme
Ø Enzyme is not directly inhibited by the end product
Ø End product reduces the concentration of enzyme by inhibiting the synthesis of new enzyme molecules
Ø Best example is the reduction of HMG CoA reductase enzyme by dietary cholesterol
(5). Isoenzyme (isozyme)
How isoenzymes regulate enzymatic activity?
 Ø Not a direct method of enzyme regulation
Ø Not a direct method of enzyme regulation
Ø Isozymes are enzyme doing similar catalytic function but having different amino acid sequences
Ø They have different kinetic parameters
Ø The Km values, Vmax and Vo of isozymes varies
Ø Km denotes the affinity of enzyme towards its substrate
Ø Isozymes enable the cell to catalyze same reaction in different conditions of the cells
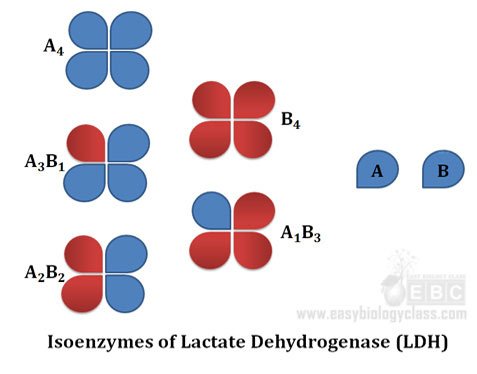
Ø Mammalian Lactate dehydrogenase (LDH) is a classic example for isoenzyme
Ø Mammalian LDH is a tetramer of two types subunits (A subunit and B subunit)
Ø LDH exists in five different forms based on the tetrameric association of A and B subunits
Ø They are A4, A3B1, A2B2, A1B3 and B4
Ø Kinetic parameters of all these isoforms varies
Ø Different tissues express different isoenzymes appropriate to their metabolic needs
Ø By regulating the relative amounts of A and B subunits the cells of various tissues can regulate the enzymatic activity in specific tissues
Ø Example: in the liver the A4 type of LDH predominates where as in heart the B4 type predominates. In brain the A1B3 type is the most common one
