Methods & Applications of Enzyme & Whole Cell Immobilization
(Advantages and Disadvantages of Enzyme Immobilization; Matrix/Supports Used in Enzyme Immobilization)
What is enzyme immobilization?
Immobilization is defined as the imprisonment of cell or enzyme in a distinct support or matrix. The support or matrix on which the enzymes are immobilized allows the exchange of medium containing substrate or effector or inhibitor molecules. The practice of immobilization of cells is very old and the first immobilized enzyme was amino acylase of Aspergillus oryzae for the production of L-amino acids in Japan.
Advantages of immobilized enzymes:
(1). Increased functional efficiency of enzyme
(2). Enhanced reproducibility of the process they are undertaking
(3). Reuse of enzyme
(4). Continuous use of enzyme
(5). Less labour input in the processes
(6). Saving in capital cost and investment of the process
(7). Minimum reaction time
(8). Less chance of contamination in products
(9). More stability of products
(10). Stable supply of products in the market
(11). Improved process control
(12). High enzyme substrate ratio
Disadvantages of enzyme immobilization:
(1). Even though there are many advantages of immobilized enzymes, there are some disadvantages also.
(2). High cost for the isolation, purification and recovery of active enzyme (most important disadvantage)
(3). Industrial applications are limited and only very few industries are using immobilized enzymes or immobilized whole cells.
(4). Catalytic properties of some enzymes are reduced or completely lost after their immobilization on support or carrier.
(5). Some enzymes become unstable after immobilization.
(6). Enzymes are inactivated by the heat generated in the system
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Applications of enzyme immobilization:
(1). Industrial production: Industrial production of antibiotics, beverages, amino acids etc. uses immobilized enzymes or whole cells.
(2). Biomedical applications: Immobilized enzymes are widely used in the diagnosis and treatment of many diseases. Immobilized enzymes can be used to overcome inborn metabolic disorders by the supply of immobilized enzymes. Immobilization techniques are effectively used in drug delivery systems especially to oncogenic sites.
(3). Food industry: Enzymes like pectinases and cellulases immobilized on suitable carriers are successfully used in the production of jams, jellies and syrups from fruits and vegetables.
(4). Research: A Research activity extensively uses many enzymes. The use of immobilized enzyme allow researcher to increase the efficiency of different enzymes such as Horse Radish Peroxidase (HRP) in blotting experiments and different Proteases for cell or organelle lysis.
(5). Production of bio-diesel from vegetable oils.
(6). Waste water management: treatment of sewage and industrial effluents.
(7). Textile industry: scouring, bio-polishing and desizing of fabrics.
(8). Detergent industry: immobilization of lipase enzyme for effective dirt removal from cloths.
Supports or Matrix used in immobilization technology:
The matrix or support immobilizes the enzyme by holding it permanently or temporarily for a brief period of time. There are a wide variety of matrixes or carriers or supports available for immobilization. The matrix used should be cheap and easily available. Their reaction with the components of the medium or with the enzyme should be minimum as possible. The matrixes or supports for immobilization of enzymes or whole cells are grouped into three major categories
(1). Natural polymers
(2). Synthetic polymers
(3). Inorganic materials
(1). Natural polymers:
(a). Alginate: A natural polymer derived from the cell wall of some algae. Calcium or magnesium alginate is the most commonly used matrix. They are inert and have good water holding capacity.
(b). Chitosan and chitin: They are structural polysaccharides occurring naturally in the cell wall of fungi and the exoskeleton of Arthropods. The various functional groups in enzymes can bind to the – OH group of chitin and can form covalent bonds.
(c). Collagen: It is the protenaceous support with good porosity and water holding capacity. The side chains of the amino acids in the collagen and that of enzyme can form covalent bonds to permanently hold the enzyme to the support.
(d). Carrageenan: It is a sulfated polysaccharide obtained from some red algae. Their good gelling properties together with its high protein holding capacity makes it good support for immobilizing enzymes.
(e). Gelatin: Gelatin is the partially hydrolyzed collagen with good water holding capacity.
(f). Cellulose: Most abundant polymer of nature and it is the cheapest support available as carrier of enzymes. The hydroxyl group of the monomer units (glucose) can form covalent bonds with that of the amino acids of enzyme.
(g). Starch: A natural polymer of amylose and amylo-pectin. It has good water holding capacity.
(h). Pectin: It is a structural polysaccharide of plants found in their primary cell wall and they also acts as the inter-cellular cementing material in plant tissues. Pectin is a gelling agent with good water holding capacity.
(2). Synthetic polymers:
They are ion exchange resins or polymers and are insoluble supports with porous surface. Their porous surface can trap and hold the enzymes or whole cells. Example: Diethylaminoethyl cellulose (DEAE cellulose), Polyvinyl chloride (PVC), UV activated Polyethylene glycol (PEG)
(3). Inorganic materials:
(a). Zeolites: They are microporous, aluminosilicate minerals with good adsorbing properties and extensively used for immobilizing enzymes and whole cells.
(b). Ceramics: They are nonmetallic solids consisting of metal and nonmetal atoms held in ionic and covalent bonds. The composition and bonding pattern varies with different types.
(c). Diatomaceous earth: They are silicious sedimentary rocks formed by fossilized accumulations of the cell wall of diatoms. Celite is the trade name of diatomaceous earth. It is a good adsorbent and are resistant to high pH and temperature.
(d). Silica:
(e). Glass:
(f). Activated carbon
(g). Charcoal
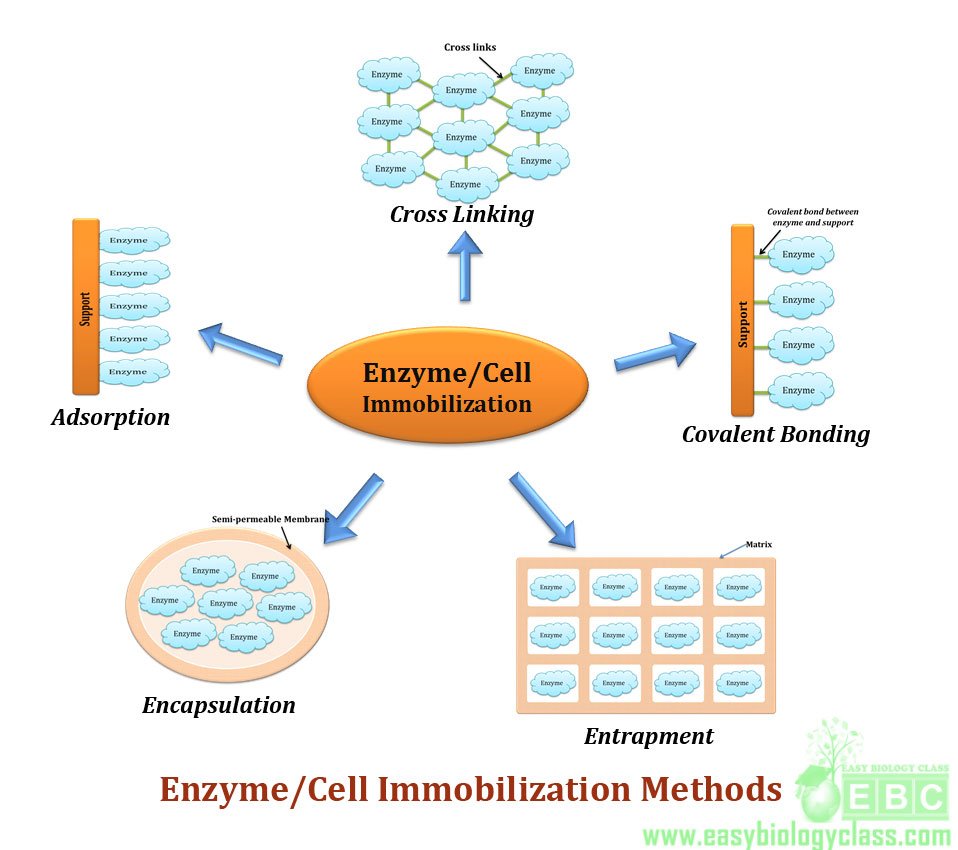
Methods of Immobilization:
Based on support or matrix and the type of bonds involved, there are five different methods of immobilization of enzyme or whole cells.
(1). Adsorption
(2). Covalent bonding
(3). Entrapment
(4). Copolymerization
(5). Encapsulation
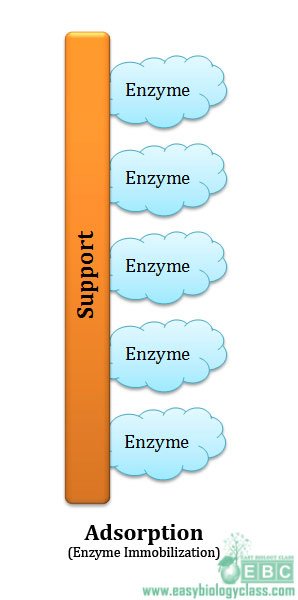 (1). Adsorption
(1). Adsorption
Adsorption is the oldest and simplest method of enzyme immobilization. Nelson & Griffin used charcoal to adsorb invertase for the first time in 1916. In this method enzyme is adsorbed to external surface of the support. The support or carrier used may be of different types such as:
(1). Mineral support (Eg. aluminum oxide, clay)
(2). Organic support (Eg. starch)
(3). Modified sepharose and ion exchange resins
There is no permanent bond formation between carrier and the enzyme in adsorption method. Only weak bonds stabilize the enzymes to the support or carrier. The weak bonds (low energy bonds) involved are mainly:
(a). Ionic interaction
(b). Hydrogen bonds
(c). Van der Waal forces
| You may also like... | ||
|---|---|---|
| NOTES | QUESTION BANK | COMPETITIVE EXAMS. |
| PPTs | UNIVERSITY EXAMS | DIFFERENCE BETWEEN.. |
| MCQs | PLUS ONE BIOLOGY | NEWS & JOBS |
| MOCK TESTS | PLUS TWO BIOLOGY | PRACTICAL |
For significant surface bonding the carrier particle size must be small (500 Å to 1 mm diameter). The greatest advantage of adsorption method is that there will not be “pore diffusion limitations” since enzymes are immobilized externally on the support or the carrier.
Methods of adsorption:
(1). Static process: Immobilization to carrier by allowing the solution containing enzyme to contact the carrier without stirring.
(2). Dynamic batch process: Carrier is placed in the enzyme solution and mixed by stirring or agitation.
(3). Reactor loading process: Carrier is placed in the reactor, and then the enzyme solution is transferred to the reactor with continuous agitation.
(4). Electrode position process: Carrier is placed near to an electrode in an enzyme bath and then the current is put on, under the electric field the enzyme migrates to the carrier and deposited on its surface.
Advantages of adsorption method:
(a). No pore diffusion limitation
(b). Easy to carry out
(c). No reagents are required
(d). Minimum activation steps involved
(e). Comparatively cheap method of immobilization
(f). Less disruptive to enzyme than chemical methods
Disadvantages of adsorption method:
(a). Desorption of enzymes from the carrier
(b). Efficiency is less
(2). Covalent bonding:
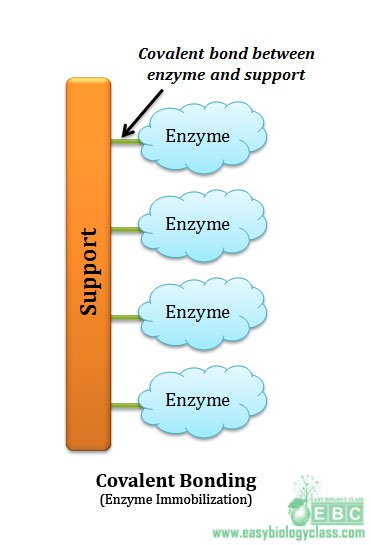 This method involves the formation of covalent bonds between the chemical groups in enzyme and to the chemical groups on the support or carrier. It is one of the widely used methods of enzyme immobilization. Hydroxyl groups and amino groups of support or enzyme form covalent bonds more easily. Chemical groups in the support or carrier that can form covalent bonds with support are amino groups, imino groups, hydroxyl groups, carboxyl groups, thiol groups, methylthiol groups, guanidyl groups, imidazole groups and phenol ring.
This method involves the formation of covalent bonds between the chemical groups in enzyme and to the chemical groups on the support or carrier. It is one of the widely used methods of enzyme immobilization. Hydroxyl groups and amino groups of support or enzyme form covalent bonds more easily. Chemical groups in the support or carrier that can form covalent bonds with support are amino groups, imino groups, hydroxyl groups, carboxyl groups, thiol groups, methylthiol groups, guanidyl groups, imidazole groups and phenol ring.
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Important functional groups of the enzyme that provide chemical groups to form covalent bonds with support or carrier are:
1. Alpha carboxyl group at ‘C’ terminal of enzyme
2. Alpha amino group at ‘N’ terminal of enzyme
3. Epsilon amino groups of Lysine and Arginine in the enzyme
4. β and γ carboxyl groups of Aspartate and Glutamate
5. Phenol ring of Tyrosine
6. Thiol group of Cysteine
7. Hydroxyl groups of Serine and Threonine
8. Imidazole group of Histidine
9. Indole ring of Tryptophan
Carriers or supports commonly used for covalent bonding are:
(a). Carbohydrates: Eg. Cellulose, DEAE cellulose, Agarose
(b). Synthetic agents: Eg. Polyacrylamide
(c). Protein carriers: Collagen, Gelatin
(d). Amino group bearing carriers: Eg. amino benzyl cellulose
(e). Inorganic carriers: Porous glass, silica
(f). Cyanogen bromide (CNBr)-agarose and CNBr Sepharose
Methods of covalent bonding
(1). Diazoation: Bonding between amino group of support and tyrosil or histidyl group of enzyme.
(2). Peptide bond: Bonding between amino or carboxyl groups of the support and that of the enzyme.
(3). Poly functional reagents: Use of a bi-functional or multifunctional reagent (glutaraldehyde) which forms covalent bonds between the amino group of the support and amino group of the enzyme.
Advantages of covalent bonding:
(a). Strong linkage of enzyme to the support
(b). No leakage or desorption problem
(c). Comparatively simple method
(d). A variety of support with different functional groups available
(e). Wide applicability
Disadvantages of covalent bonding (major problem with covalent bonding):
(a). Chemical modification of enzyme leading to the loss of functional conformation of enzyme.
(b). Enzyme inactivation by changes in the conformation when undergoes reactions at the active site. This can be overcome through immobilization in the presence of enzyme’s substrate or a competitive inhibitor.
(3). Entrapment:
In this method enzymes are physically entrapped inside a porous matrix. Bonds involved in stabilizing the enzyme to the matrix may be covalent or non-covalent. The matrix used will be a water soluble polymer. The form and nature of matrix varies with different enzymes. Pore size of matrix is adjusted to prevent the loss of enzyme. Pore size of the matrix can be adjusted with the concentration of the polymer used. Agar-agar and carrageenan have comparatively large pore sizes. The greatest disadvantage of this method is that there is a possibility of leakage of low molecular weight enzymes from the matrix.
Examples of commonly used matrixes for entrapment are:
(1). Polyacrylamide gels
(2). Cellulose triacetate
(3). Agar
(4). Gelatin
(5). Carrageenan
(6). Alginate
Methods of entrapment:
(a). Inclusion in the gels: enzymes trapped inside the gels.
(b). Inclusion in fibers: enzymes supported on fibers made of matrix material.
(c). Inclusion in microcapsules: Enzymes entrapped in microcapsules formed by monomer mixtures such as polyamine and calcium alginate.
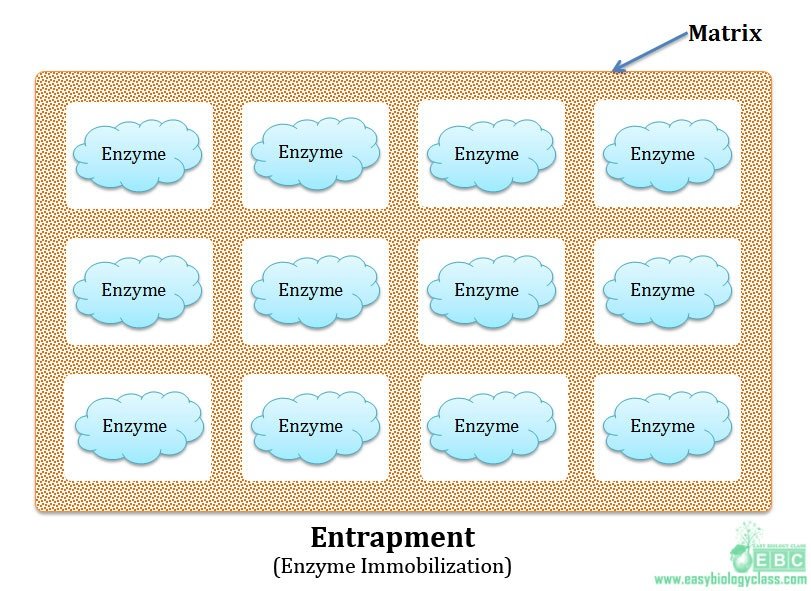
Advantages of entrapment:
(a). Fast method of immobilization
(b). Cheap (low cost matrixes available)
(c). Easy to practice at small scale
(d). Mild conditions are required
(e). Less chance of conformational changes in enzyme
(f). Can be used for sensing application
Disadvantages of entrapment:
(a). Leakage of enzyme
(b). Pore diffusion limitation
(c). Chance of microbial contamination
(d). Not much success in industrial process
(4). Cross linking (copolymerization):
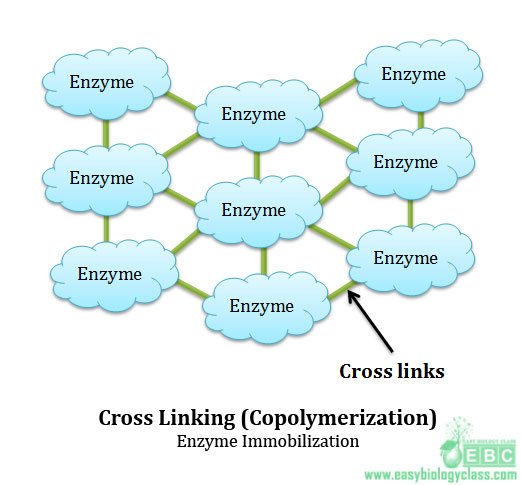 This method is also called as copolymerization. In this method of immobilization enzymes are directly linked by covalent bonds between various groups of enzymes via polyfunctional reagents. Unlike other methods, there is no matrix or support involved in this method. Commonly used polyfunctional reagents are glutaraldehyde and diazonium salt. This technique is cheap and simple but not often used with pure enzymes. This method is widely used in commercial preparations and industrial applications. The greatest disadvantage or demerit of this method is that the polyfunctional reagents used for cross linking the enzyme may denature or structurally modify the enzyme leading to the loss of catalytic properties.
This method is also called as copolymerization. In this method of immobilization enzymes are directly linked by covalent bonds between various groups of enzymes via polyfunctional reagents. Unlike other methods, there is no matrix or support involved in this method. Commonly used polyfunctional reagents are glutaraldehyde and diazonium salt. This technique is cheap and simple but not often used with pure enzymes. This method is widely used in commercial preparations and industrial applications. The greatest disadvantage or demerit of this method is that the polyfunctional reagents used for cross linking the enzyme may denature or structurally modify the enzyme leading to the loss of catalytic properties.
(5). Encapsulation:
This type of immobilization is done by enclosing the enzymes in a membrane capsule. The capsule will be made up of semi permeable membrane like nitro cellulose or nylon. In this method the effectiveness depends upon the stability of enzymes inside the capsule.
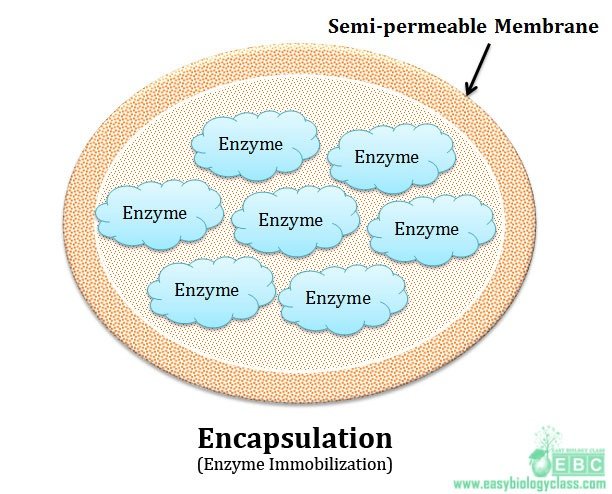
Advantages of encapsulation:
(a). Cheap and simple method
(b). Large quantity of enzymes can be immobilized by encapsulation
Disadvantages of encapsulation:
(a). Pore size limitation
(c). Only small substrate molecule is able to cross the membrane
| You may also like NOTES in... | ||
|---|---|---|
| BOTANY | BIOCHEMISTRY | MOL. BIOLOGY |
| ZOOLOGY | MICROBIOLOGY | BIOSTATISTICS |
| ECOLOGY | IMMUNOLOGY | BIOTECHNOLOGY |
| GENETICS | EMBRYOLOGY | PHYSIOLOGY |
| EVOLUTION | BIOPHYSICS | BIOINFORMATICS |
Immobilization of whole cells:
Immobilization of whole cells is an alternative to enzyme immobilization and it is a well-developed method for the utilization of enzymes from microbes. Immobilization of whole cells become particularly effective when the individual enzymes become inactive during direct immobilization, or the isolation and purification of enzyme is not cost effective. The greatest advantage of whole cell immobilization is that here the enzymes will be active and stable for long period of time since they are in their natural environment. Use of immobilized cells for fermentation is a very old practice. Bacteria or yeast cells are immobilized by adsorbing it on woodchips. This is practiced in many parts for different types of fermentations.
Advantages of whole cell immobilization:
(a). Multiple enzymes can be introduced to a single step
(b). Extraction and purification of enzymes are not required
(c). Enzymes are stable for long time
(d). Native conformation of enzyme is best maintained
(e). Cell organelles like mitochondria and chloroplasts can be immobilized
(f). Cost effective method
Disadvantages of whole cell immobilization:
(a). Concentration of enzymes will be less
(b). Production of unwanted enzymes and unwanted products
(c). Modification of end products by other enzymes produced by immobilized cells
Methods of immobilization of whole cells:
Methods of immobilization of whole cells are same as that described for enzyme immobilization and they include:
1. Adsorption
2. Covalent bonding
3. Cell to cell cross linking
4. Encapsulation
5. Entrapment
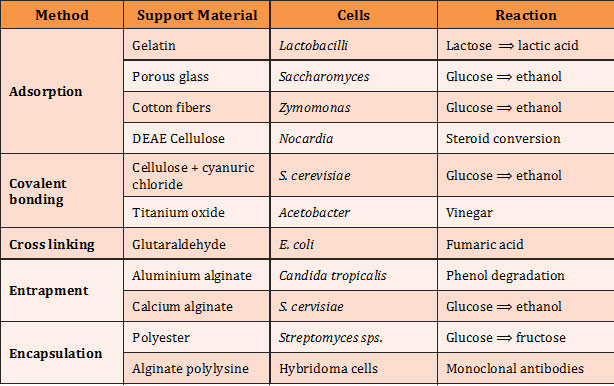
Immobilization of cells: Methods, Support materials, Cells and Reaction: