Diffusion vs Osmosis
Osmosis and Diffusion Difference: Diffusion and Osmosis are the two methods in membrane transport by which the movement of materials in and out of the cell takes places.
Diffusion: Diffusion is the movement of particles from a region of higher concentration to a region of lower concentration. Example: When a scent-bottle is opened in a room, quickly the fragrance will spread to the entire room by diffusion of the molecules through the air.
You may also like NOTES in...
BOTANY BIOCHEMISTRY MOL. BIOLOGY
ZOOLOGY MICROBIOLOGY BIOSTATISTICS
ECOLOGY IMMUNOLOGY BIOTECHNOLOGY
GENETICS EMBRYOLOGY PHYSIOLOGY
EVOLUTION BIOPHYSICS BIOINFORMATICS

Osmosis: Osmosis is a type of diffusion in which the solvent molecules move into the solution through a semi-permeable membrane. Example: Plasmolysis of a cell when it is placed in a sugar or salt solution.
The present post describes the Differences between Diffusion and Osmosis with a Comparison Table.
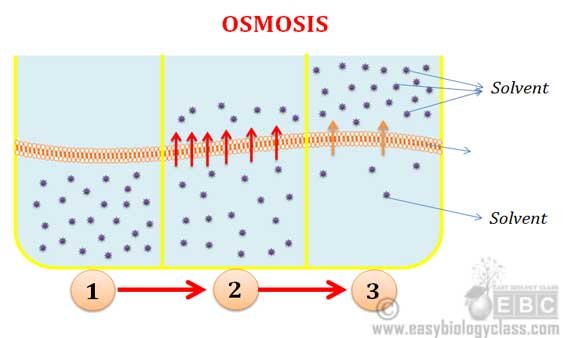
Osmosis and Diffusion Difference
Sl. No. Diffusion Osmosis
1 Definition: Movement of particles from a region of higher concentration to a region of lower concentration. Definition: Movement of solvent molecules to solution through a semi-permeable membrane.
2 Example: Diffusion of ink in water Example: Plasmolysis of a cell in salt solution.
3 No membrane is required for the diffusion process. Osmosis can take place only through a semi-permeable membrane.
4 Diffusion can occur between any medium such as gas to gas, liquid to liquid, liquid to gas, solid to gas and solid to liquid. Osmosis can occur only in liquid medium.
5 In diffusion, both solvent and solute molecules can diffuse. In osmosis, only the solvent (not the solute) molecules can diffuse.
6 Diffusion can occur between similar or dissimilar types of solvents. The osmosis can take place only between similar types of solvents.
7 In diffusion, the flow of particles can occur in all directions. In osmosis, the flow particles can occur only in one direction.
8 The diffusion process can neither be stopped nor reversed. Osmosis can be reversed or stopped by applying additional pressure on the solution side.
9 Diffusion is NOT influenced by the solute potential. Osmosis is influenced by the solution potential.
10 Diffusion depends on the free energy of different particles. Osmosis depends on the rate of reduction of free energy of one solvent.
11 At the end of diffusion process, the concentration of the diffusing particles will be equalized. At the end of osmosis, the concentration of solvent will not be equalized
12 Diffusion is not influenced by the turgour pressure. Osmosis is hindered by the turgour pressure of the cell.
<< Back to Plant Physiology Notes Page

Thanks so much sir
Thank you
Keep visiting easybiologyclass
Regards
Thank you so much
A.a .
It gives us extra information about boilogy chapters which was not provided to us at school.
Thnx
Yaar ghazab bro bhut hi ghazab ka website hai tumhara than you
Very very helpful in the study of science … Because studY materials that provided by easy biology is very easy to understand also very helpful to teacher to explain……. Yash
Thank you Mr. Yash Verma