Alpha Rays vs Beta Rays vs Gamma Rays
An unstable atomic nuclei loss its energy by emitting radiations such as alpha rays, beta rays and gamma rays by a process called radioactive decay. A substance with such an unstable nucleus is called the radioactive substance.
The particles produced by radioactive decay, i.e., alpha particles, beta particles and gamma rays are considerably different with distinct physical, chemical and biological properties.
Alpha rays
They are also called alpha particles. Alpha rays consist of two protons and two neutrons bound tougher into particles. It is identical to the helium nucleus. Alpha particles are produced as a result of the alpha decay of a radioactive material such as Uranium-238.
Beta rays
They are also called beta particles. Beta rays are high energy high and speed electrons emitted from a radioactive material after the beta decay. Potassium-40 is a beta emitter.
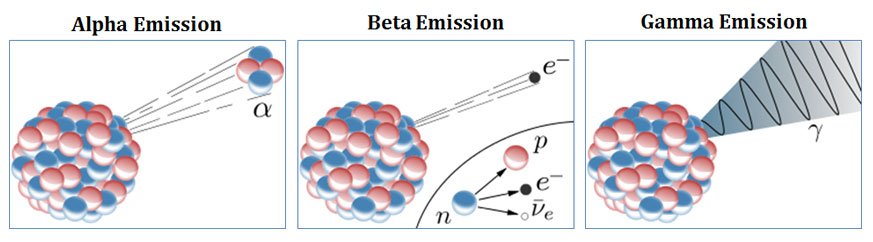
image source: wikipedia
Gamma rays
They are also called gamma radiations. Gamma radiations are electromagnetic radiations with high energy and high penetration capacity produced from a radioactive material after the gamma decay. Radium-226 is a gamma emitter.
The following table shows the similarities and differences between alpha particles, beta particles and gamma rays.
Difference between Alpha Beta and Gamma Rays
Propertis Alpha Rays Beta Rays Gamma Rays
Symbol α β γ
Mass 6.65 X 10^-27 kg 5.5 x 10^-4 amu Negligible
Charge 2 positive charge 1 negative charge No charge
Speed and Nature High speed helium nucleus High speed electrons High speed electromagnetic radiations
Velocity ~5% of the velocity of light Nearly equal to that of light Equal to the velocity of light
Penetration power Low Moderate, 100 times more than alpha particles High, 100 times more than beta particles
Effect of magnetic and electric field Deflected towards the negative late Deflected towards the positive plate Not deflected
Ionizing power Greater than beta and gamma rays Very low Very low
Luminescence Produce fluorescence and phosphorescence Produce phosphorescence Produce phosphorescence
Distance travelled 2 – 4 cm 2 – 3 meters 500 meters
You might also like…
@. Properties of Alpha, Beta and Gamma Rays
@. GM Counter vs Proportional Counter
@. GM Counter vs Scintillation Counter


Thanks for the post.