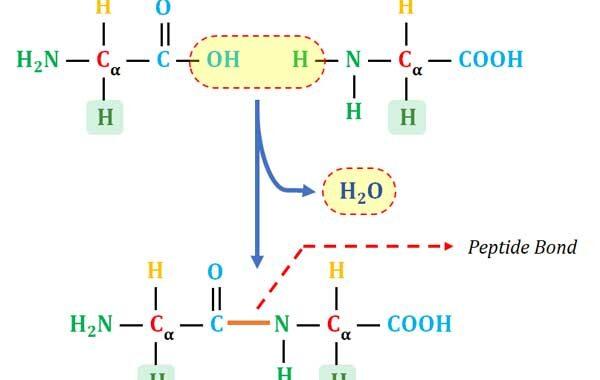
Learning Objectives: What are Proteins? How Proteins are Formed? What is Peptide Bond? Peptide vs Protein, C-Terminal and N-Terminal of Proteins, pI of Proteins, Sub-units of Proteins and Characteristics of Peptide Bond What are Proteins? Ø Proteins are the polymers of amino acids (specifically the polymers of L-α- amino acids). […]
Continue ReadingCategory Archives: Biochemistry
easybiologyclass tutorials in biochemistry
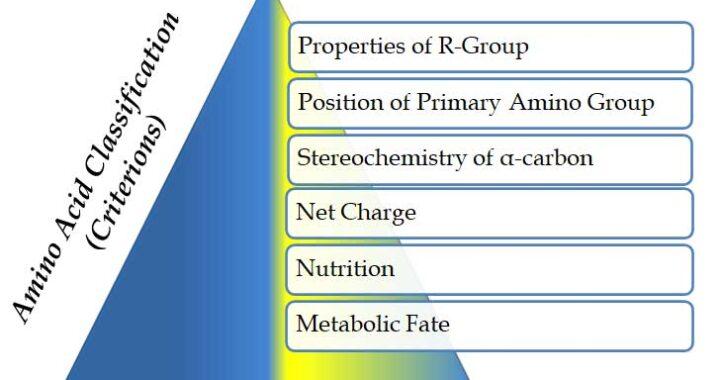
Amino Acid Classification (Biochemistry Notes)
In the previous post, we discussed the Structure of Amino acids. There we have discussed the characteristics of amino acids and their ionic behavior. Here, we will discuss Classification of Amino Acids in Biochemistry. Amino acids are classified based on different criterions such as properties of R-group, nutrition, metabolic fate […]
Continue ReadingAmino Acid: Structure and Functions (Biochemistry Short Notes)
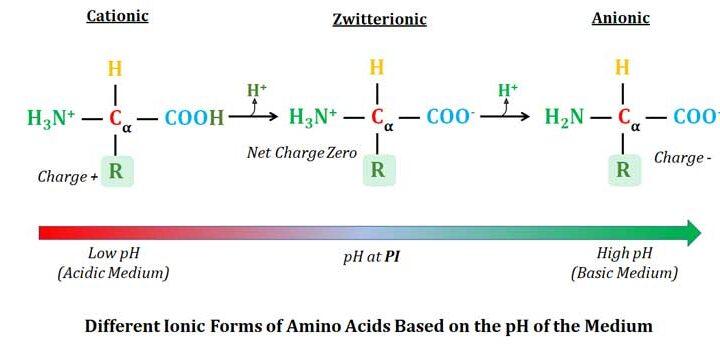
Learning Objectives- Amino Acids: Structure and Functions: What are Amino Acids? List of Amino Acids, Three Letter Code and Single Letter Code of Amino Acids, Discovery of Amino Acids, Structure of Amino Acids, Proteogenic Amino Acids and Non Proteogenic Amino Acids, Alpha Amino Acids and Beta Amino Acids, Isomerism in […]
Continue ReadingGATE XL Biochemistry Mock Test-07
Dear GATE XL Aspirants, Welcome to your GATE XL Biochemistry Mock Test – 07. This Mock Test is for the preparation of GATE Life Sciences (XL) Biochemistry Examination. It Consists of 10 Questions in MCQ format. Once you finish the test, please click ‘SUBMIT‘ button to see your ‘SCORE‘ and ‘CORRECT […]
Continue ReadingGATE XL Mock Test (Biochemistry-06)
Dear GATE XL Aspirants, Welcome to your GATE XL Biochemistry Mock Test – 06. This Mock Test is for the preparation of GATE Life Sciences (XL) Biochemistry Examination. It Consists of 10 Questions in MCQ format. Once you finish the test, please click ‘SUBMIT‘ button to see your ‘SCORE‘ and ‘CORRECT […]
Continue Reading